Low Grade Endometrial Stromal Sarcoma with Smooth Muscle Differentiation, Associated with Grade 1 Endometrioid Endometrial Adenocarcinoma: A Case Report

Global Journal of Pathology & Laboratory Medicine
Volume 1, Issue 3, October 2021, Pages: 1-9
Received: September 01, 2021; Reviewed: September 04, 2021; Accepted: September 13, 2021; Published: October 12, 2021
Unified Citation Journals, Pathology 2021, 1(2) 1-08; https://doi.org/10.52402/Pathology208
ISSN 2754-0952
Authors: Dr. Ravindra Sawant, Dr. Ayesha Ajmi, Dr. Nadia Ali-Ross
Royal Bolton Hospital, Bolton NHS foundation trust, UK
Download PDF1. Abstract:
We are presenting a case of low-grade endometrial stromal sarcoma with smooth muscle differentiation and associated with grade 1 endometrioid endometrial adenocarcinoma. Smooth muscle differentiation in endometrial stromal sarcoma is a known entity but this in combination with an endometrioid type endometrial adenocarcinoma, has been rarely reported in the literature. Few publications and less than 10 case reports have been noted in the literature review.
Preoperative radiology investigations were not able to differentiate between leiomyoma and an endometrial stromal sarcoma. The diagnostic endometrial biopsy was reported as grade 1 endometrioid endometrial adenocarcinoma. A total abdominal hysterectomy with bilateral salpingo-ophorectomy was performed. The final histology diagnosis was confirmed based on immunohistochemistry and associated genetic alterations. There has been no reported recurrence over a period of 1 year.
2. Introduction:
WHO (2014) classifies endometrial stromal tumours into endometrial stromal nodule (ESN), low-grade endometrial stromal sarcoma (LGESS), high-grade endometrial stromal sarcoma (HGESS), and undifferentiated uterine sarcoma (UUS). Although endometrial stromal tumors represent the second most common category of mesenchymal tumors of the uterus, they are rare and account for less than 1% of all uterine tumours.1
Low-grade endometrial stromal sarcomas account for approximately 0.2–1% of all uterine malignancies and 6–20% of all uterine sarcomas, representing the second most common uterine sarcoma. Patients typically present in the pre or perimenopausal age with a median age of 52 years, with a uterine mass and abnormal uterine bleeding.
Low-grade endometrial stromal sarcomas are typically indolent tumours with an overall survival 70-84% and 65-76% 5 and 10-year survival rate. 1 Collision tumours in the uterus are rare. The prognosis in the case of endometrial stromal sarcomas depends on the stage. The associated adenocarcinoma generally tends to be grade 1 endometrioid adenocarcinoma, similar to the current case.
Case presentation:
A 54 years old woman presented with postmenopausal bleeding over 2 weeks and a history of menopause four years prior. She was known to have uterine fibroids causing some pressure symptoms but had previously declined hysterectomy or embolization. Her medical history included mild asthma and previous excision of Basal Cell carcinoma from her eyelid and stripping of varicose veins. She had a normal BMI of 24kg/m2 and a large fibroid uterus equivalent to a 20-week size pregnancy. Her cervix was stenosed but appeared normal.
Initial investigations:
An ultrasound scan of the pelvis showed a bulky uterus with a 101x93x87 mm large posterior/fundal fibroid and a thickened endometrium of 25mm. She underwent an outpatient hysteroscopy which revealed polypoid, vascular, and thickened endometrium which was suspicious of endometrial cancer. An endometrial biopsy was taken, and an MRI scan was arranged. Endometrial biopsy confirmed FIGO grade 1 endometrioid adenocarcinoma. The MRI scan reported a large fibroid uterus with a lobulated mass in the endometrium confined to the inner myometrium, consistent with stage 1a endometrial cancer. The case was discussed at the cancer Multidisciplinary team meeting and Total Abdominal Hysterectomy and Bilateral Salpingo-Oophorectomy (TAHBSO) was advised at the local hospital. The surgery was uncomplicated.
Histopathology findings:
The specimen of the uterus with the cervix and bilateral tubes and ovaries was received.
-Macroscopic examination of the uterus revealed the following: tan to a white, polypoidal lesion within the endometrial cavity, extending into the myometrium, with no areas of haemorrhage or necrosis with thickening of the endometrial lining. The adnexae were unremarkable.
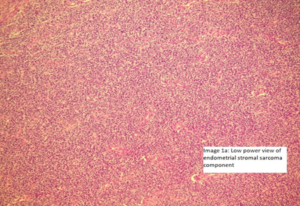
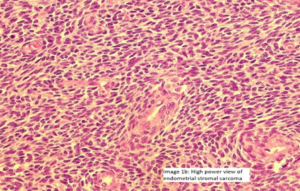
Image 1a and 1b (Low and high power view)
-Microscopic examination, Endometrial stromal sarcoma: the cells are seen in sheets or vaguely storiform pattern; they have scant cytoplasm and uniform, oval to spindle-shaped nuclei with an occasional very small nucleolus. The neoplastic cells focally whorl around small vessels reminiscent of the arterioles seen in proliferative phase endometrium
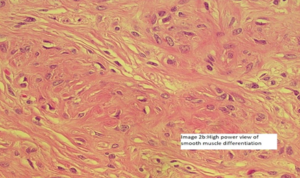
Image 2 (Smooth muscle differentiation can be seen as pale to ‘pink’ irregular islands of slightly epithelioid cells.)
Image 2a -There is a central area of hyalinization from which collagen bands radiate toward the periphery and cells with an ‘epithelioid’ appearance are embedded in collagen fibers ( Image 2a and image 2b).
Image 2b-There was mild nuclear pleomorphism and a very low mitotic count was appreciated. There were no areas with necrosis or haemorrhage. There was no lymphovascular invasion noted.
Image 3-The endometrioid type endometrial adenocarcinoma shows features consistent with Figo grade 1 (Image 3).
The carcinoma showed superficial myometrial invasion with no intravascular or perineural invasion and was staged as FIGO stage 1A.
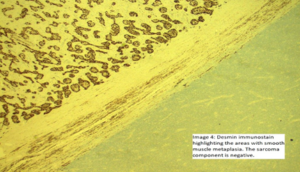
Image 4 and 5. The smooth muscle component was highlighted by using desmin and smooth muscle actin;
Image 5.
Image 6: Endometrial stromal sarcoma demonstrating a distinct area with smooth muscle differentiation.
Desmin highlighting the smooth muscle differentiation
H& E section demonstrating the distinct zonation demonstrating stromal component and adjacent smooth muscle differentiation.
CD10 immunostain: CD10 is a very helpful marker in the diagnosis of endometrial stromal tumors as they typically display diffuse and strong immunoreactivity. The lesional cells within the endometrial stromal sarcoma were diffusely positive for CD10 with staining of the smooth muscle component.
However, some tumors may only be weakly and focally positive or completely negative as typically occurs in t(10;17) endometrial stromal tumors6 and undifferentiated endometrial sarcomas of pleomorphic type and undifferentiated endometrial sarcomas of pleomorphic type.
Genetic alteration for low-grade endometrial stromal tumors is (7;17) resulting in JAZF1-SUZ12 changes were noted in the tumour, confirming the diagnosis.
Endometrial stromal tumors similar to normal endometrial stroma are surrounded by a network of reticulin fibers that can be highlighted by a reticulin stain.
Follow up:
Following the surgery and the final histology diagnosis, a baseline CT scan of the thorax, abdomen, and pelvis was performed, given the risk of metastasis with a low-grade stromal sarcoma. The tumour was limited to the uterus and was staged as stage 1b (>5cm). Her case was also discussed with the Specialist Sarcoma team as there was no clear consensus on optimal follow-up. The follow-up plan is to see her every 6-12 months in the clinic.
Discussion
- Uterine tumours exhibiting both endometrial stromal and smooth muscle differentiation are relatively rare and similar lesions have previously been designated as stromomyoma 2, a term not in use currently. Focal areas of smooth muscle differentiation are commonly seen but a criteria of >30% smooth muscle component on the H&E section (which was noted in the current case) has been defined, for the lesion to be labelled as an endometrial stromal sarcoma with smooth muscle differentiation.
- Endometrial stromal tumours can show variant features, including but not limited to, smooth muscle metaplasia, rhabdoid, skeletal muscle differentiation, adipose metaplasia, sex-cord-like elements, and occasionally bizarre nuclei. None of these benign changes appear to influence the prognosis. The prognosis of these tumours appears to relate to the margin status of the endometrial stromal component.1
- The fact that such tumours exist is not surprising because it has been suggested that multipotential cells are present in the uterus that can differentiate into the endometrial stroma and smooth muscle. 3
- It is important to note that glandular elements have been reported as part of endometrial stromal sarcomas. The glands are widely spaced, show endometrioid morphology, and display a minimal degree of cytological atypia. Foci of grade 1 endometrioid adenocarcinoma have been rarely reported. 1
- There have been cases reported with the presence of numerous benign endometrial-type glands within the neoplasm. In many areas, a “zoning” phenomenon was present, with endometrial glands surrounded by endometrial stroma, which was in turn surrounded by smooth muscle.2 In these cases it is important to note that the endometrial glands were of a benign type and part of the lesion. In our case, the glandular component was a distinct invasive carcinoma and not part of the endometrial stromal sarcoma.
- The coexistence of two adjacent and histologically distinct tumour components, endometrioid adenocarcinoma and ESS, is best defined as a collision tumour, as seen in this case. Literature review suggests all cases with such coexisting tumours, had FIGO grade 1 endometrioid adenocarcinoma, abutting but distinctly separate from the background ESS.4, similar to that seen in our case.
- It is of great importance to differentiate collision tumour from carcinosarcoma due to the impact of the diagnosis on clinical management and prognosis.
- The impact of factors such as advancing age, lymphovascular invasion, hormonal status, and ploidy are still being evaluated. In a recent study, mitotic count and tumour cell necrosis were reported to be important prognostic factors in stage I endometrial stromal sarcomas and in conjunction could be used to stratify patients with low-grade endometrial stromal sarcomas into three prognostic categories. 5
- It is important to differentiate this tumour from more aggressive tumours like carcinosarcomas, especially when the stromal sarcoma component shows high-grade features.
- A brief description of the other types of stromal tumours.
- Endometrial Stromal nodule (ESN) are benign tumours of uterus, usually presenting with abnormal vaginal bleeding or as an incidental finding in hysterectomy specimens performed for other reasons. This is the least common of all endometrial stromal tumours. Small irregularities in the form of finger-like projections or satellite islands ≤3 mm in maximum dimension and <3 in number are allowed. As endometrial stromal nodules are by definition benign, simple hysterectomy is the treatment of choice. However, a more conservative therapy may be considered in young patients who want to preserve fertility with close follow-up using a combination of ultrasound and hysteroscopy to monitor the growth of the tumor.
- High grade endometrial stromal sarcoma: This is malignant tumour of endometrial stromal derivation with high-grade, round-cell morphology sometimes associated with a low-grade spindle cell component that is most commonly fibromyxoid.7 They typically show destructive invasion of the myometrium in contrast to the permeative invasion of low-grade endometrial stromal sarcomas. The prognosis of these tumours is somewhat in between low grade endometrial stromal sarcomas and undifferentiated sarcomas.
-
- Undifferentiated endometrial sarcoma: Typically shows high-grade cytologic features without specific type of differentiation.8 They are typically intraluminal polypoid masses, usually >10 cm, with a fleshy cut surface and areas of necrosis and/or haemorrhage. Cyclin D1 can be diffusely positive but in those cases the tumors are also typically positive for CD10 (which excludes YWHAE-FAM22 sarcomas). Focal smooth muscle actin, desmin, EMA or keratin positivity may be seen. 9 It is important to rule out other sarcomas like leiomyosarcoma, rhabdomyosarcoma and carcinosarcoma.
- There have been cases reported of undifferentiated endometrial stromal sarcoma associated with grade 1 endometrioid endometrial adenocarcinoma.Microscopically, the tumor was composed of a larger component of undifferentiated stromal sarcoma that was distinct from a smaller endometrioid adenocarcinoma. 10 11
- Collision tumours associated with endometrial stromal sarcomas are uncommon and most cases have adenocarcinoma and a sarcoma as the two components. Very rarely endometrial stromal sarcoma has been reported with cervical squamous cell carcinoma. 12
Conclusion:
- The extensive (>30%) smooth muscle differentiation identified in this case is not commonly seen with ESS. The smooth muscle component demonstrated typical benign features.
- The unusual finding was that of a coexisting endometrioid endometrial adenocarcinoma. Although a rare association, the previously reported cases also had grade 1 endometrioid endometrial adenocarcinoma associated with this stromal tumour, similar to our case. 10 11 The endometrioid carcinoma was distinct from the stromal sarcoma component. Such collision tumours are exceedingly rare.
- Radical hysterectomy with bilateral salpingo-oophorectomy seems to be the treatment of choice, to avoid secondary hormonal stimulation of the tumour.
- Tumour stage is believed to be the most important factor for prognosis.
- Although both endometrial stromal sarcoma with endometrioid adenocarcinoma and carcinosarcoma, are surgically managed, adjuvant therapy may be quite different.
- Recurrences are known to occur, typically after many years following the initial diagnosis and at sites like the abdomen and even the lungs.
References:
[1]- George L Mutter, Jaime Prat: Pathology of the female genital tract, Third Edition, Chapter 20, Pages 425-436
[2] W G McCluggage, A J Cromie, C Bryson, A I Traub, Dr McCluggage: Uterine endometrial stromal sarcoma with smooth muscle and glandular differentiation Volume 54, issue 6. https://jcp.bmj.com/content/54/6/481
[3] Scully RE. Smooth-muscle differentiation in genital tract disorders. Arch Pathol Lab Med 1981:105:505–7
[4] GraceKima Huyen Q.Pham, AminRamzanb EstherElishaevc Paulette Mhawech-Faucegliab: Endometrioid adenocarcinoma associated with endometrial stromal sarcoma: A rare, often unrecognized collision tumor. Gynecologic Oncology Reports Volume 13, August 2015, Pages 8-12
[5] D’Angelo E, Ali RH, Espinosa I et al: Endometrial stromal sarcomas with sex cord differentiation are associated with PHF1 rearrangement. Am J Surg Pathol 2013;37:514–521
[6] Lee CH, Marino-Enriquez A, Ou W, et al.: The clinicopathologic features of YWHAE-FAM22 endometrial stromal sarcomas: a histologically high-grade and clinically aggressive tumor. Am J Surg Pathol. 36:641-653 2012 PMID: 22456610
[7] Kurihara S, Oda Y, Ohishi Y, et al.: Coincident expression of beta-catenin and cyclin D1 in endometrial stromal tumors and related high-grade sarcomas. Mod Pathol. 23:225-234 2010 PMID: 19898427
[8] Hendrickson MR, Tavassoli F, Kempson RL, et al.: Mesenchymal tumours and related lesions. Tavassoli F Devilee P WHO classification of tumours. Pathology and genetics of tumors of breast and female genital organs. 2003 IARC Lyon, France 233-244
[9] Kurihara S, Oda Y, Ohishi Y, et al.: Endometrial stromal sarcomas and related high-grade sarcomas: immunohistochemical and molecular genetic study of 31 cases. Am J Surg Pathol. 32:1228-1238 2008 PMID: 18580489
[10] Liu G, Zhang C, Ma Z, Zhang Q, Liu B. Int J Clin Exp Pathol. Endometrial stromal sarcoma with endometrioid adenocarcinoma of the uterus: a case report. 2015 May 1;8(5):5242-6. eCollection 2015. PMID: 26191224
[11] Celik H, Kokcu A, Yildiz L. Eur J Gynaecol Oncol. Endometrial stromal sarcoma with coexistent endometrioid adenocarcinoma in a woman with previous breast cancer: a preliminary case report. 2013;34(5):493-5. PMID: 24475593
[12] Tanveer N, Gupta B, Pathre A, Rajaram S, Goyal N. A Rare Collision Tumour of Uterus- Squamous Cell Carcinoma and Endometrial Stromal Sarcoma. J Clin Diagn Res. 2017 Feb;11(2):ED20-ED22. doi: 10.7860/JCDR/2017/23532.9405. Epub 2017 Feb 1. PMID: 28384878
© Copyright 2021, All Rights Reserved. Use of this content signifies your agreement to the T&Cs of Unified Citation Journals
This abstract of Manuscript/Paper/Article is an open access Manuscript/Paper/Article distributed under the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/) which allows and permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited and accepted.
This communication and any documents, or files, attached to it, constitute an electronic communication within the scope of the Electronic Communication Privacy Act (https://it.ojp.gov/PrivacyLiberty/authorities/statutes/1285)
To citation of this article: Dr. Ravindra Sawant, Dr. Ayesha Ajmi, Dr. Nadia Ali-Ross, Low Grade Endometrial Stromal Sarcoma with Smooth Muscle Differentiation, Associated with Grade 1 Endometrioid Endometrial Adenocarcinoma: A Case Reports, Global Journal of Pathology & Laboratory Medicine