Plasticity of Gastric Tumor-Initiating Cells
![Figure 8 Summary of cell plasticity models [22]](https://ucjournals.com/wp-content/uploads/2021/10/Figure-8-Summary-of-cell-plasticity-models-22.png)
Global Journal of Pathology & Laboratory Medicine
Volume 1, Issue 2, April 2021, Pages: 58-71
Received: August 20, 2021; Reviewed: September 15, 2021; Accepted: October 05, 2021; Published: October 12, 2021
Unified Citation Journals, Pathology 2021, 1(2) 1-08; https://doi.org/10.52402/Pathology207
ISSN 2754-0952
Authors: Mrs. Andreea Nelson Twakor![]()
Faculty of Medicine, “Ovidius” University, Constanta, Aleea Universitatii nr. 1, 900470, Constanta, Romania
Download PDFKeywords: Adenocarcinoma, Abnormal Chromatin, Gastric Cancer, Plasticity
1. Abstract:
Gastric cancer is the world’s 5th largest cause of cancer mortality (7.7% of all cancer deaths) and the 6th most common cancer (11.1% of all cancers) [1]. The human body contains up to two meters of DNA in each cell if stretched end to end. That DNA must be compressed inside the cell nucleus in order to fit [2]. The packing of the genome structure, which is the chromatin, determines how cells respond to stress, according to research in this area. A cell’s plasticity increases when its chromatin packing is disorganized and cannot respond as quickly to outside stimuli when the chromatin is tidy and organized [3].
In this study, the investigation is twofold. The association between the degree of plasticity and abnormal chromatin in cancer patients was first explored, with a focus on individuals with stomach cancers.
The adaptability of tumor cells and their relationships with normal stem cells were studied in depth. Second, stomach metaplasia is linked to tissue atrophy and the loss of the parietal cells that secret acid. This is expected to cause the formation of a metaplastic mucous cell lineage with high amounts of TFF2 (spasmolytic polypeptide), which has been associated with gastric adenocarcinoma [4].
Normal stem cells that get activated in response to unexpected or unfavorable physiological conditions may be the source of cancer stem cells [5]. Thus, the findings of this study suggest that cancer cells are masters of change, the ability given by the relation between abnormal chromatin and tissue exposure to outside conditions.
2. Background:
A combination of environmental variables and the accumulation of certain genetic mutations cause gastric cancer. Healthy eating habits, anti-H. pylori medications, chemoprevention, and early detection screening are all part of the primary prevention strategy [6].
More than half of all new cases are seen in underdeveloped nations. Between the greatest and lowest-risk populations, there is a 20 fold difference in risk. A high incidence in cases is found in Eastern Europe, China and Japan, Central and South America, while regions such as Australia, North America, New Zeeland are considered zones with a low incidence [8].
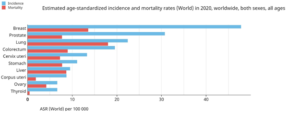
Figure 1: International Agency for Research on Cancer [7]
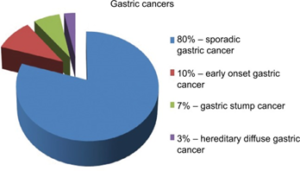
Figure 2: Gastric cancer: epidemiology [9]

Figure 3: Gastric cancer risk factors [10]
3. Research objectives:
This research is looking at different scenarios or cell plasticity as well as molecular mechanisms of gastric carcinogenesis.
Cancer cell plasticity has been considered as a key mechanism that supports cancer cell diversity and contributes to intra-tumor heterogeneity, along with genetic and epigenetic changes. Cancer cells with plasticity have the ability to go back and forth between a differentiated state with reduced tumorigenic potential and an undifferentiated or cancer stem-like state (CSC), which is responsible for long-term tumor formation. This plasticity also grants the potential for transition into several CSC conditions that have varying levels of ability to invade, disseminate, and seed metastasis [11]. The epithelial-to-mesenchymal transition has been related to cancer cell plasticity, which depends on one hand to cell-autonomous pathways, but on the other hand, on signals delivered by the tumor microenvironment which are produced as a reaction to therapy.
In this study, the research focuses on the transition for cancer cell states, the mechanisms behind cell plasticity, and how this affects tumor growth. It is important to address all these issues as gaining a better insight into the principles underlying cancer cell adaptability helps researchers to develop novel therapeutic approaches [12].
4. Methodology:
For this study, we used literature review as a research method. The research is disparate and interdisciplinary, and it included information from research articles in Pathology, Molecular Biology, and Genetics fields.
This research provides a broader look by summarizing and integrating the different guidelines when it comes to plasticity in cancer cells by building on and synthesizing these diverse forms of literature evaluations. The goal of this study is to give an overview of and suggestions for various forms of literature reviews as a research approach in stomach cancer research.
5. Histology and Pathology
Gastric cancer (GC) is highly heterogeneous histologically, both architecturally and cytologically, with many histologic features frequently coexisting. The classification of GC includes an early or an advanced stage, in order to help select the best course of treatment, as well as histologically into subtypes based on the predominant morphologic component. When a tumor is found in the proximal stomach or cardia, it might be challenging to classify it based on anatomic location, especially if the tumor also includes the gastroesophageal junction (GEJ) [13].
6. Macroscopy
GCs are classified into three kinds by endoscopists based on their endoscopic appearance. According to Díaz Del Arco et al. (2021), there are three types: protruded (type I), pedunculated (Ip), and sessile (Is); superficial (type II), and excavated (type III). As it is the most common (with 80% of cases) a further sub-classification is offered for type II: raised (IIa), flat (IIb), and depressed (IIc), with the latter being the most prevalent [14].
Advanced GC can display various gross appearances. The most extensively used classification is the one offered by Bormann, which separates GC into polypoid carcinoma (type I), fungating carcinoma (type II), ulcerated carcinoma (type III), and diffusely infiltrative cancer (type IV) [13].
![Figure 4 Growth patterns and macroscopic appearance of advanced gastric cancer according to the Bormann classification [13]](https://ucjournals.com/wp-content/uploads/2021/10/Figure-4-Growth-patterns-and-macroscopic-appearance-of-advanced-gastric-cancer-according-to-the-Bormann-classification-13-300x140.png)
Figure 4: Growth patterns and macroscopic appearance of advanced gastric cancer according to the Bormann classification [13]
6. Macroscopy
Gastric cancer is divided into two categories, according to Lauren’s criteria: intestinal and diffuse. The epidemiology, and pathology of these two types differ significantly [15].
Tubular, papillary, mucinous, and poorly cohesive (including signet ring cell carcinoma) are the four major histologic patterns of gastric malignancies recognized by 2010 WHO classification, as well as unusual histologic variants. The classification is based on the carcinoma’s most prominent histologic pattern, which frequently coexists with less prominent features of other histologic patterns [16].
![Figure 5 Representative histological photographs (H&E) of human gastric cancer [17]](https://ucjournals.com/wp-content/uploads/2021/10/Figure-5-Representative-histological-photographs-HE-of-human-gastric-cancer-17-300x74.png)
Figure 5: Representative histological photographs (H&E) of human gastric cancer [17]
7. Plasticity
Tumor heterogeneity has posed an enduring barrier to the development of cancer medicines in recent decades. This tumor heterogeneity can be inter-tumoral, in which patients with different tumor types have different tumor genotypes, or intra-tumoral, in which cancer cells within the same tumor have different phenotypic and functional heterogeneity. Genetic variety, changes in gene regulation, transitions between cellular states, and environmental disturbances can all contribute to intra-tumor heterogeneity. Cancer progression and treatment failure are fueled by heterogeneity, which makes prognosis difficult and encourages disease recurrence. To explain intra-tumor heterogeneity, two models were presented at first [18].
The first model looks at the clonal evolution model. It entails intrinsic variations between cancer cells induced by stochastic genetic and/or epigenetic changes in individual cells. Over time, cancer cells collect these changes, and better-adapted clones with a growth advantage are chosen. Clonal advantage can vary over time and space. Indeed, extrinsic mechanisms, such as those provided by the different microenvironments within a tumor, confer functional differences upon cancer cells at these different locations [18].
The second model is the cancer stem-like cell (CSC) model. This theory is based on the idea that a small group of cells known as cancer stem cells is responsible for tumor formation. CSCs have self-renewing ability, initiate and maintain long-term tumor development, as opposed to most tumor cells, which are based on a non-CSC approach, they have a more differentiated phenotype [19].
In an experiment conducted in 2018 which was performed on mouse models, Victoria da Silva-Diz et al. concluded that adult stem cells with certain mutations are at the origin of skin, gastric and cerebral tumors and that these transformed stem cells to act as CSCs [20]. However, other studies have reached the conclusion that CSCs could also originate from other cells by dedifferentiation or reprogramming processes [21].
The third model recently suggested, refers to the cancer cells that have the dynamic ability to shift between non-CSC and CSC states, as dictated by intrinsic and extrinsic stimuli. Various investigations have supported the idea that CSCs are dynamic populations capable of spontaneous state shifts, and a population of non-stem cells was found to spontaneously switch CSC in both vitro and vivo [22].
Cell plasticity refers to a cell’s capacity to change its phenotype as a reaction to external stimuli, without undergoing genetic changes. Increased plasticity has been linked to pathological diseases, particularly neoplasms. Barrett’s esophagus, which is a pre-malignant state of esophageal adenocarcinoma, was cited by Shen et. al as an example of plasticity, as it involves the transformation of esophageal squamous lining into an intestinal-like columnar epithelium [23].
![Figure 6 Cancer cell plasticity Impact on tumor progression and therapy response [12]](https://ucjournals.com/wp-content/uploads/2021/10/Figure-6-Cancer-cell-plasticity-Impact-on-tumor-progression-and-therapy-response-12-300x150.png)
Figure 6: Cancer cell plasticity: Impact on tumor progression and therapy response [12]
Molecular Mechanisms of Gastric Carcinogenesis
Environmental variables such as H. pylori infection and nutrition, as well as the accumulation of generalized and specific genetic changes, are thought to cause GC. Tahara and Yasui created a model that summarizes the sequence of molecular events for intestinal type and diffuse type GC [23]. The intestinal type of gastric adenocarcinoma is preceded by a sequence of histological lesions (known as Correa´s cascade) with well-defined characteristics: non-atrophic gastritis → multifocal atrophic gastritis without metaplasia → intestinal metaplasia of the complete type → intestinal metaplasia of the incomplete type→ dysplasia [24].
As one can see from this model, there are certain alterations which are common to both major histological subtypes of GC, such as p53 mutation, cyclin E overexpression/amplification, or aberrant CD44 transcripts. Others like KRAS mutations, CDH1 mutations, and amplifications of HER2 FGFR2, and MET appear to be more ‘specific’ for one of the histological subtypes [25].
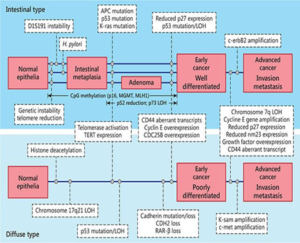
Figure 7 The Yasui Tahara multistep model of molecular pathogenesis of gastric cancer [25]
![Figure 8 Summary of cell plasticity models [22]](https://ucjournals.com/wp-content/uploads/2021/10/Figure-8-Summary-of-cell-plasticity-models-22-300x267.png)
Figure 8: Summary of cell plasticity models [22]
Results
Tumor cells have been found to interfere with normal cell developmental processes in order to adapt to environmental challenges. Shen et. al (2020) presented four scenarios of cell plasticity that reflect the challenges that tumor cells are facing and overcoming at different levels, starting from within the cell and finishing at a systemic level [23].
At the first level, the epigenetic regulators and DNA elements found in the chromatin influence tumor cell tolerance to chemotherapy. Intracellular signaling paths that detect a variety of environmental signals modify the transcriptional landscape, resulting in tumor cells adapting to medical treatment. At the microenvironmental level, there are inter-cellular networks of communications. Shen et. al conclude that these direct cell-to-cell interactions help build up a “safe heaven” [23]. Combined with quorum sensing mechanisms, tumor cells develop stress adaptation methods. At a systemic level, tumor-derived neurotrophins trigger the neurogenic switch, which attracts tumor adjacent fibers and neural precursor cells. As a result, nerve fibers that surround the tumor help cancer cells to spread [27].
Chromatin level
Other kinds of cellular plasticity may be vital for organismal survival following injury when it is critical to swiftly regenerate and restore tissue integrity. Changes in the epigenetic landscape of tissues are anticipated to occur in these circumstances in order to facilitate typically limited cell fate changes. Epigenetic processes, notably DNA methylation and histone changes, have been demonstrated to play a key role in controlling plasticity in the brain [28].
The reversible aspect of cell plasticity prompted by medical treatment suggests that transcriptional regulation is a key factor at an epigenetic level. Histone lysine demethylase 5 (KDM5) may be one of the most investigated epigenetic regulators in cancer cell plasticity [23]. In several tumors, lysine-specific demethylase 5A (KDM5A) is linked to cancer development, differentiation, multi-drug resistance, invasion, and metastasis. In gastric adenocarcinoma, KDM5A represses CDKIs (p16, p21, and p27) and transactivates VEGF. It promotes tumor growth, metastasis, and angiogenesis [29].
Cell level: intracellular regulatory pathways
G proteins may be directly or indirectly linked to receptors via most cell surface receptors, which stimulate intracellular target enzymes. These intracellular enzymes act as downstream signaling elements, propagating and amplifying the ligand-binding-induced signal. Intracellular signal transduction, in most cases, entails a sequence of processes that send signals from the cell membrane to a variety of intracellular targets. Transcription factors, which regulate gene expression, are frequent targets of such signaling cascades. Intracellular signaling pathways connect the nucleus to the cell membrane, causing a change in the gene expression once there is a change in the external environment [30].
Microenvironmental level: tumor–stroma interactions
Tumor cells and stromal cells, such as fibroblasts, endothelial cells, and infiltrating immune cells, make up solid tumors. The tumor microenvironment, which includes embedded extracellular matrix and vascularization, is involved not only in tumor development but also in plasticity produced by drug therapy.
CAFs (cancer-associated fibroblasts) is one of the most well-studied cell types when it comes to generating therapeutic resistance. In BRAF wild-type melanoma associated fibroblasts, BRAF inhibition causes a contradictory stimulation of the MAPK/ERK pathway. These cells, in turn, cause increased integrin 1-FAK-Src activation in melanoma cells that are being treated, resulting in a drug-tolerant situation that provides the tumor cells with a “safe heaven” [23].
Systemic Level: Neuronal Factors
The emotional discomfort and psychiatric disorders are common among cancer patients throughout their treatment, resulting in systemic release of neuroendocrine hormones and neurotransmitters that may alter the tumor`s micro and macroenvironments. TRPA1, a redox-sensing Ca 2+-influx channel found in neurons, has been demonstrated to regulate Ca 2+-dependent anti-apoptotic pathways and protect cancer cells from chemotherapy, suggesting that cancer cells may tolerate chemotherapy-induced oxidative stress by delivering a pain signal. Targeted treatments have also documented system level regulators of tumor adaptive response to anti-cancer therapy. [23].
Conclusion
Cancer cells exhibit great plasticity in most tumors, which endows them with the ability to shift dynamically and reversibly between non-CSC and CSC state. Furthermore, CSCs may also transit between states, exhibiting distinct features and abilities to disseminate and give rise to metastatic lesions, which may influence cancer progression and therapy response. This dynamic activity has been linked to the activation of the EMT program, in certain cancers.
Activation of this program results in a reversible switch of phenotypic features, which encompass a spectrum of cells, from epithelial to mesenchymal-like cancer (stem-like) cells, as well as intermediates states, in which cells conserve epithelial features, but also express mesenchymal markers.
Cancer cell plasticity is influenced by signals given by the tumor microenvironment in response to treatment, as well as cancer cell-autonomous processes such as genetic and epigenetic modifications. EMT program is controlled by the complex integration of multiple regulatory networks, which include epigenetic modifications, transcriptional control, and activation of specific signaling pathways.
Thus, the expression of EMT-TFs is modulated by DNA methylation, histone modifications, including the establishment of bivalent chromatin that primes gene expression to respond rapidly to stimuli, miRNAs, and factors that indirectly regulate these events. In turn, EMT-TFs inhibit the expression of epithelial genes and induce the expression of mesenchymal state genes by driving the recruitment of DNA methylation and histone modifier enzymes to the promoter of target genes. Furthermore, cytokines and growth factors provided by CAFs, mesenchymal stem cells, endothelial cells, and determined immune cells, as well as hypoxia, can trigger transcriptional and epigenetic programs, leading to the acquisition of plasticity, induction of stemness and (partial) EMT.
Therefore, integrating all these mechanisms that operate at different levels may give rise to intermediate states or hybrid epithelial/mesenchymal cells, which show a strong capacity to adapt rapidly to different stresses during tumor growth or in response to therapy. This extraordinary capacity to adapt to microenvironmental changes is an important challenge to therapeutic interventions. Therefore, further studies are needed to provide insight into the mechanisms that regulate cancer cell plasticity, in order to design new therapeutic interventions targeted to block EMT/stemness or to eradicate mesenchymal or hybrid cancer cells, acting on cancer cells or the tumor microenvironment.
References:
[1] International Agency for Research on Cancer. (2021, 07 10). Cancer today. Retrieved from www.gco.iarc.fr:https://gco.iarc.fr/today/online-analysis-multi-bars?v=2020&mode=cancer&mode_population=countries&population=900&populations=900&key=asr&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=10&
[2] Alberts B, J. A. (2002). Chromosomal DNA and Its Packaging in the Chromatin Fiber (4th edition ed.). New York: Garland Science. Retrieved 11 07, 2021, from https://www.ncbi.nlm.nih.gov/books/NBK26834/
[3] Virk, R. K. (2020). Disordered chromatin packing regulates phenotypic plasticity. Science Advances, Volume 6(2), eaax6232. doi:10.1126/sciadv.aax6232
[4] Elise S. Hibdon, L. C. (2018). Cellular plasticity in the stomach: insights into the cellular origin of gastric metaplasia. Gastroenterology, 154(4), 801-803. doi:10.1053/j.gastro.2018.02.001
[5] Kiyoung Eun, S. W. (2017). Cancer stem cell heterogeneity: origin and new perspectives on CSC targeting. BMB Rep, 50(3), 111-125. doi:10.5483/BMBRep.2017.50.3.222
[6] Sitarz, R., Skierucha, M., Mielko, J., Offerhaus, G., Maciejewski, R., & Polkowski, W. P. (2018). Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer management and research, 10, 239–248. https://doi.org/10.2147/CMAR.S149619
[7] International Agency for Research on Cancer
[8] Parkin, D.M., Bray, F., Ferlay, J. and Pisani, P. (2005), Global Cancer Statistics, 2002. CA: A Cancer Journal for Clinicians, 55: 74-108. https://doi.org/10.3322/canjclin.55.2.74
[9]Sitarz, R., Skierucha, M., Mielko, J., Offerhaus, G., Maciejewski, R., & Polkowski, W. P. (2018). Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer management and research, 10, 239–248. https://doi.org/10.2147/CMAR.S149619
[10] Gomceli, Ismail & Demiriz, Baris & Tez, Mesut. (2012). Gastric carcinogenesis. World journal of gastroenterology : WJG. 18. 5164-70. 10.3748/wjg.v18.i37.5164.
[11] Easwaran, H., Tsai, H. C., & Baylin, S. B. (2014). Cancer epigenetics: tumor heterogeneity, plasticity of stem-like states, and drug resistance. Molecular cell, 54(5), 716–727. https://doi.org/10.1016/j.molcel.2014.05.015
[12] da Silva-Diz V, Lorenzo-Sanz L, Bernat-Peguera A, Lopez-Cerda M, Muñoz P. Cancer cell plasticity: Impact on tumor progression and therapy response. Semin Cancer Biol. 2018 Dec;53:48-58. doi: 10.1016/j.semcancer.2018.08.009. Epub 2018 Aug 18. PMID: 30130663.
[13] Irene Gullo, Fátima Carneiro, Gastric Cancer: Pathology and Genetics, Encyclopedia of Cancer (Third Edition), Academic Press, 2019, Pages 77-98, ISBN 9780128124857, https://doi.org/10.1016/B978-0-12-801238-3.65076-6
[14] Díaz Del Arco, C., Ortega Medina, L., Estrada Muñoz, L., Molina Roldán, E., Cerón Nieto, M. Á., García Gómez de Las Heras, S., & Fernández Aceñero, M. J. (2021). Are Borrmann’s Types of Advanced Gastric Cancer Distinct Clinicopathological and Molecular Entities? A Western Study. Cancers, 13(12), 3081. https://doi.org/10.3390/cancers13123081
[15] Ma, J., Shen, H., Kapesa, L., & Zeng, S. (2016). Lauren classification and individualized chemotherapy in gastric cancer. Oncology letters, 11(5), 2959–2964. https://doi.org/10.3892/ol.2016.4337
[16] Hu, B., El Hajj, N., Sittler, S., Lammert, N., Barnes, R., & Meloni-Ehrig, A. (2012). Gastric cancer: Classification, histology and application of molecular pathology. Journal of gastrointestinal oncology, 3(3), 251–261. https://doi.org/10.3978/j.issn.2078-6891.2012.021
[17] Echizen, Kanae & Hirose, Osamu & Maeda, Yusuke & Oshima, Masanobu. (2016). Inflammation in gastric cancer: Interplay of the COX-2/PGE 2 and TLR/MyD88 pathways. Cancer Science. 107. n/a-n/a. 10.1111/cas.12901.
[18]Victoria da Silva-Diz, Laura Lorenzo-Sanz, Adrià Bernat-Peguera, Marta Lopez-Cerda, Purificación Muñoz, Cancer cell plasticity: Impact on tumor progression and therapy response, Seminars in Cancer Biology, Volume 53, 2018, Pages 48-58, https://doi.org/10.1016/j.semcancer.2018.08.009.
[19] Yoo, M. H., & Hatfield, D. L. (2008). The cancer stem cell theory: is it correct?. Molecules and cells, 26(5), 514–516.
[20] Zhang, Y., Zeng, F., Han, X. et al. Lineage tracing: technology tool for exploring the development, regeneration, and disease of the digestive system. Stem Cell Res Ther 11, 438 (2020). https://doi.org/10.1186/s13287-020-01941-y
[21] Friedmann-Morvinski, D., & Verma, I. M. (2014). Dedifferentiation and reprogramming: origins of cancer stem cells. EMBO reports, 15(3), 244–253. https://doi.org/10.1002/embr.201338254
[22] Chaffer C. L., Brueckmann I., Scheel C., Kaestli A. J., Wiggins P. A., Rodrigues L. O., et al. (2011). Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc. Natl. Acad. Sci. U.S.A. 108 7950–7955. 10.1073/pnas.1102454108
[23] Shen, S., & Clairambault, J. (2020). Cell plasticity in cancer cell populations. F1000Research, 9, F1000 Faculty Rev-635. https://doi.org/10.12688/f1000research.24803.1
[24] Yasui W, Oue N, Aung PP, Matsumura S, Shutoh M, Nakayama H. Molecular-pathological prognostic factors of gastric cancer: a review. Gastric Cancer. 2005;8(2):86-94. doi: 10.1007/s10120-005-0320-0. PMID: 15864715.
[25] Piazuelo, M. B., & Correa, P. (2013). Gastric cáncer: Overview. Colombia medica (Cali, Colombia), 44(3), 192–201.
[26] Grabsch HI, Tan P. Gastric cancer pathology and underlying molecular mechanisms. Dig Surg. 2013;30(2):150-8. doi: 10.1159/000350876. Epub 2013 Jul 18. PMID: 23867592.
[27] Cervantes-Villagrana, R. D., Albores-García, D., Cervantes-Villagrana, A. R., & García-Acevez, S. J. (2020). Tumor-induced neurogenesis and immune evasion as targets of innovative anti-cancer therapies. Signal transduction and targeted therapy, 5(1), 99. https://doi.org/10.1038/s41392-020-0205-z
[28] Paksa, A., & Rajagopal, J. (2017). The epigenetic basis of cellular plasticity. Current opinion in cell biology, 49, 116–122. https://doi.org/10.1016/j.ceb.2018.01.003
[29] Yang, GJ., Zhu, MH., Lu, XJ. et al. The emerging role of KDM5A in human cancer. J Hematol Oncol 14, 30 (2021). https://doi.org/10.1186/s13045-021-01041-1
[30] Cooper GM. The Cell: A Molecular Approach. 2nd edition. Sunderland (MA): Sinauer Associates; 2000. Pathways of Intracellular Signal Transduction. Available from: https://www.ncbi.nlm.nih.gov/books/NBK9870/
© Copyright 2021, All Rights Reserved. Use of this content signifies your agreement to the T&Cs of Unified Citation Journals
This abstract of Manuscript/Paper/Article is an open access Manuscript/Paper/Article distributed under the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/) which allows and permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited and accepted.
This communication and any documents, or files, attached to it, constitute an electronic communication within the scope of the Electronic Communication Privacy Act (https://it.ojp.gov/PrivacyLiberty/authorities/statutes/1285)
To citation of this article: Mrs. Andreea Nelson Twakor, Plasticity of Gastric Tumor-Initiating Cells, Global Journal of Pathology & Laboratory Medicine
Tags
Breast Pathology Journals | Breast Inflammation Journals | Pathologist Journals | Surgical Pathology Journals | E-Pathology Journals | Anatomic Pathology Journals | Histopathology Journals | Cytopathology Journals | Thyroid Gland Pathology | Cancer Pathology Journals | Oncology Journals | Surgical Pathology Journals

