Cervico Facial Actinomycotic Osteomyelitis : A Single Center Experience
Global Journal of Pathology & Laboratory Medicine
Volume 2, Issue 1, July, 36-46
Unified Citation Journals, Pathology 2023, 2(1) 36-46; https://doi.org/10.52402/Pathology219
ISSN 2754-0952
Author: Dr. Vinila Belum Reddy 1, Dr M Naveen Kumar 2, Dr S Jayabhaskar Reddy 3, Dr Manimekhala P 4, Dr Vanajakshi S 5
1) Assistant Professor, Department Of Pathology, AIMSR Hyderabad
2) Assistant Professor, Department Of Pathology, AIMSR Hyderabad
3) Professor & Hod, Department Of Pathology, AIMSR Hyderabad
4) Professor Department Of Pathology, AIMSR Hyderabad
5) Assistant Professor, Department Of Statistics, AIMSR Hyderabad
INTRODUCTION
Actinomycosis is an infrequent invasive bacterial disease that has been recognized for over a century. It is a chronic granulomatous suppurative and fibrosing disease caused by gram-positive, non acid fast anaerobic to microaerophilic, branched, and filamentous bacteria mainly belonging to the human commensal flora of the oropharynx, gastrointestinal tract, and urogenital tract. A. israelii accounts for 52% of the infections, whereas Actinomyces viscosus, Actinomyces odontolyticus, Arachnia propionica and Actinomyces meyeri contribute to 40%, 5%, 2%, 2% and 1%, respectively. (1-3)
Multiple different clinical features of actinomycosis have been described, as various anatomical sites (such as face, bone and joint, respiratory tract, genitourinary tract, digestive tract, central nervous system, skin, and soft tissue structures) can be affected.In any site, actinomycosis frequently mimics malignancy, tuberculosis, or nocardiosis, as it spreads continuously and progressively, and often forms a cold abscess.Actinomycotic infection has a typical subacute to chronic clinical course primarily afflicting soft tissues and rarely the bones. Characteristically, a breach in the integrity of mucous membranes by preceding infection, trauma, or the surgery may help bacteria gain entry to deeper body structures and lead to disease progression. Predominantly, the spread of infection is by direct invasion and rarely by the hematogenous or metastatic spread.(1-5)
The most common presentation is cervicofacial, which accounts for over half of the reported cases. Actinomyces israelii is found to be primarily associated with the cervicofacial actinomycosis. Infection of the mandible is most common, accounting for 53.6% of the total, followed by cheek (16.4%), Chin (13.8%), Maxilla (5.7%) and Temporomandibular Junction (0.3%). Actinomycosis involving the maxilla is rare accounting for 0.5-9% of all head and neck cases.(1-6)
AIMS AND OBJECTIVES
The aim of this study is to comprehensively analyze histopathologic specimens of patients diagnosed with Cervicofacial Actinomycosis Osteomyelitis at Apollo Institute of Medical sciences and Research.
MATERIALS AND METHODS
The project will be along the lines of an observational study, conducted over a period of one and half year at the Pathology laboratory of Apollo Institute of Medical Sciences and Research, a tertiary care teaching hospital located in Hyderabad. This is an Retrospective observational time bound descriptive study being done in the Department of Pathology, Apollo Institute of Medical Sciences and Research, Hyderabad, Telangana from January 2021 to June 2022 wherein histopathological and clinical data of all the patients who underwent Cervicofacial Actinomycosis Osteomyelitis were retrieved and analyzed.
The gross specimens will be retrieved from the museum and records will be assessed for the type of grossing method employed. The H&E slides will be reassessed. Additionally, tissue sections will be stained with Giemsa, PAS, GMS, and Ziehl Neelsen stains, as required. Descriptive analysis of the data involved will be calculated using percentage, mean, median and range. Appropriate statistical methods will be applied as needed. In this observational study we intend to exhibit our experience with this rarity and document its pathological presentation.
CASE SERIES AND PATIENTS
After institutional review board approval, a retrospective review of our pathological data base was performed to identify patients with pathologically proven Actinomycosis Osteomyelitis between January 2021 to June 2022 and November. Both the history and the chief presenting complaint were evaluated. Histologic diagnosis was established after surgical excision in all patients.
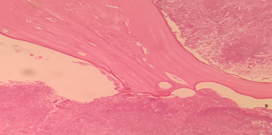
Case 1
18 /Male presented with swelling in the palate. Examination revealed palatal swelling extending from central incisor to premolar region with the possibility of Radicular cyst or Dentigerous cyst. Macroscopic examination revealed multiple discharging sinuses from the mandible. Palatal Swelling biopsy showed Radicular cyst with Actinomycotic Osteomyelitis.
Figure 1:
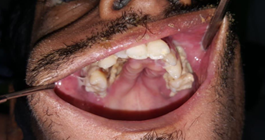
Case 2
Figure 2:
30/Male, is a case of suspected fungal osteomyelitis of Left maxilla. Macroscopic examination received multiple bony soft tissue bits measuring 3,5x3x2 cms
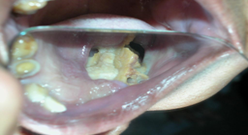
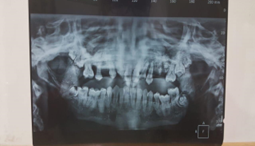
Case 3
Figure 3:
25/Male presented with pyogenic osteomyelitis. Macroscopic examination revealed multiple discharging sinuses from the mandible.
DISCUSSION AND REVIEW OF THE LITERATURE
In 1877, pathologist Otto Bollinger described the presence of A. bovis in cattle, and shortly afterwards, James Israel discovered A. israelii in humans. In 1890, Eugen Bostroem isolated the causative organism from a culture of grain, grasses, and soil. After Bostroem’s discovery, a general misconception existed that actinomycosis was a mycosis that affected individuals who chewed grass or straw. (7) Actinomyces resembles both bacteria and fungi, thus they were often considered to be transitional between the two groups. However, most of the fundamental characteristics of Actinomyces indicate that they are, in fact, bacteria.[8]
Cope in 1938 suggested that the infection may be classified anatomically as cervicofacial (i.e. lumpy jaw), thoracic or abdominal. Cervicofacial is the most common among all and accounts for more than half of the reported cases.(9) Although rarely seen, due to its aggressive and locally destructive nature, actinomycosis of the oral cavity is a highly significant condition.(10) In cervicofacial actinomycosis which is most frequent, the mandible is more commonly involved than maxilla (4:1). It requires a break in the integrity of the mucous membranes and the presence of devitalized tissue to invade deeper body structures and to cause disease which occurs through oro-maxillofacial trauma, dental extractions, dental caries or most probably through any dental intervention as the causative organism.(9)
Osteomyelitis is inflammation of the bone and its marrow contents. Changes in the calcified tissues are secondary to inflammation of the soft tissue component of the bone. Actinomycosis rarely affects the bone. Osteomyelitis due to actinomyces has been reported infrequently.The spread of Actinomyces by hematogenous route with intraosseous granuloma formation and minimal subperiosteal bone reaction has been reported by Bala et al.(11) Mandibular actinomycotic osteomyelitis is usually underappreciated by many clinicians in their assessment of head and neck infections. Most of the cases are traced to an odontogenic source with periapical tooth abscess and post traumatic or surgical complication as the key antecedent events.(12) From the past 48 years period, a systematic search of MEDLINE data base was performed and reported 30 cases of Actinomycosis Osteomyelitis affecting the jaws.(8) Actinomyces bacteria enters through the Jaw fracture, oral surgery, an infected tooth socket, deep periodontal pockets, and a root canal and leads to the consequent development of Actinomycosis.(8)
Therefore, the prerequisite for the development of endogenous disease is the transport of pathogens into tissue layers with an anaerobic environment.(13) These organisms lack tissue decomposing enzymes (hyaluronidases), therefore, they require the aid of other accompanying bacterial flora to achieve pathogenicity. The presence of accompanying bacteria, particularly streptococci and staphylococci, having a synergistic effect in the pathogenicity of cervicofacial actinomycosis.(14,15) Although the pathogenesis of actinomycosis osteomyelitis is unclear, it is suggested that inflammation begins when the normal composition of the microbial flora is disturbed, and chronic inflammation leads to localized pathological changes in the bone. It is assumed that the mandibular predominance of the disease stems from the relatively poor vascularization of the condensed cortical bone in the mandible with a similar mechanism that predisposes it to osteoradionecrosis.(16)
Overview of the different species of Actinomyces bacteria that cause actinomycosis
Bacteria of the genus Actinomyces belong to the Actinobacteria phylum and Actinomycetales order and are related to other genera such as Corynebacterium, Mycobacterium, Nocardia, and Propionibacterium.(1-5) Besides Actinomyces, Propionibacterium propionicum (formerly Arachnia propionica), has often been reported as an agent of actinomycosis-like infections. More than 30 species of Actinomyces have been described. Actinomyces israelii is the most prevalent species isolated in human infections and is found in most clinical forms of actinomycosis. Actinomyces viscosus and Actinomyces meyeri are also often reported in typical actinomycosis, although they are less common, and A. meyeri is considered to have a great propensity for dissemination. Some species, including Actinomyces naeslundii, Actinomyces odontolyticus, Actinomyces gerencseriae (formerly A. israelii serotype 2), Actinomyces neuii, Actinomyces turicensis, and Actinomyces radingae, have been associated with particular clinical syndromes. Thus, A. israelii and A. gerencseriae are responsible for about 70% of orocervicofacial infections.(1-5) Hematogenous dissemination of actinomycosis is extremely rare and has mainly been associated with A. meyeri, A. israelii, and A. odontolyticus.(17)
Of note, most of the Actinomyces spp. are present in polymicrobial flora. Therefore Actinomycesare often isolated with other normal commensals, such as Aggregatibacter actinomycetemcomitans, Eikenella corrodens, Capnocytophaga, fusobacteria, Bacteroides, staphylococci, streptococci, or Enterobacteriaceae, depending on the site of infection.(2)
As such, it is difficult to discriminate colonization of mucosa-contaminating samples and infection due to Actinomyces except when the culture is pure and associated with the presence of polynuclear neutrophils. On the other hand, Actinomyces infections could be polymicrobial and associated with other bacteria, named “companion microbes”, which contribute to initiation and development of infection by inhibiting host defenses or reducing oxygen tension.(4) The multimicrobial nature of infection is well described in animal models and in human cervicofacial actinomycosis.(18)
Microbiological diagnosis of actinomycosis
The bacteriological identification of Actinomyces from a sterile site confirms the diagnosis of actinomycosis. However, isolation and identification of these causative bacteria occur in only a minority of cases; the failure rate of culture is high because of previous antibiotic therapy, inhibition of Actinomyces growth by concomitant and/or contaminant microorganisms, inadequate culture conditions, or inadequate short-term incubation.(19) Because of the microaerophilic or strict anaerobic character of Actinomyces, strict anaerobic processing (rapid transport to the laboratory and/or transport in an anaerobic transport medium) and anaerobic growth conditions should be used for primary isolation.
The most appropriate clinical specimens are tissue from surgical biopsy or pus; swabs must be avoided. Finally, clinicians should indicate suspicion for actinomycosis to the microbiologist to ensure that prolonged culture on appropriate media and in an appropriate atmosphere is performed. Moreover, the identification of Actinomyces in mucosa, where these bacteria are normal inhabitants, is of little significance in the absence of sulfur granules or a typical clinical syndrome, highlighting the importance of microbiological investigations in combination with histologic analysis.
A Gram stain of the specimen is usually more sensitive than culture, especially if the patient had received antibiotics. Actinomyces are non-spore-forming Gram-positive rods. Except for A. meyeri, which is small and nonbranching, all the other species are branching filamentous rods.
Growth of Actinomyces is slow; it appears within at least 5 days and may take up to 15–20 days. Thus, incubation of at least 10 days is required before conclusion of a negative culture. Most Actinomyces spp. are facultative anaerobes, but some relevant species (such as A. meyeri), are strictly anaerobic, so cultures must be incubated in an anaerobic atmosphere. Actinomyces can be cultured on chocolate blood agar media at 37°C. Other enriched media can be used for Actinomyces isolation: brain heart infusion broth and Brucella Blood Agar with hemin and vitamin K1. The use of semi-selective media (such as phenylethyl alcohol or mupirocin-metronidazole blood agar) may increase isolation rates by inhibiting overgrowth of concomitant organisms.(20)
Actinomyces can be initially suspected by colony morphology and biochemical profiling. For example, A. israelii forms a “molar tooth” colony on agar and grows as clumps within broth, whereas A. odontolyticus forms rust-brown or red-colored colonies.
Actinomyces are indole-negative bacteria. Identification was classically based on phenotypic tests (urease, catalase, fermentation of sugars, etc) or on commercial biochemical kits but, in fact, such tests can lead to misidentification of species and even of genus.(21-22) Serological assays have been developed but need to be improved before they become clinically useful.
The classification of Actinomyces spp., initially based on phenotypic characters, has been recently revised, due to advances in microbiological taxonomy that use genotypic methods such as comparative 16S ribosomal RNA (rRNA) gene sequencing. Therefore, nowadays, molecular techniques such as 16S rRNA sequencing serve as the reference for identification.(23) Another practical identification method consists of 16S ribosomal DNA restriction analysis.(24) Polymerase chain reaction with specific primers can also be used for direct detection of Actinomyces In clinical material.
Matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) should be a quicker and accurate tool for Actinomyces identification in the future. Mass spectrometry uses an ionization source to charge and separate ionized bacterial proteins; then, a detector and mass analyzer generate a mass spectrum specific to bacterial species. Concerning Actinomyces, MALDI-TOF allows accurate identification at the genus level, but species identification remains uncertain and depends on the mass spectrometry system and the species studied.(23) Improvement of this technique is needed for a definitive identification of Actinomyces spp., but it so far seems very promising.
Pathology of actinomycosis
Gram staining of pus and pathology of infected tissue is of great interest for the diagnosis of actinomycosis, as it is usually more sensitive than culture, which remains sterile in more than 50% of cases. Once Actinomyces spp. have invaded tissues, they develop a chronic granulomatous infection characterized by the formation of tiny clumps, called sulfur granules because of their yellow color. These formations of 0.1–1 mm in diameter, composed of an internal tangle of mycelial fragments and a rosette of peripheral clubs, are stabilized by a protein–polysaccharide complex, which is supposed to provide a resistance mechanism to host defenses by inhibiting phagocytosis.(25-28) Histopathology examination discloses one to three sulfur granules in about 75% of cases, described as basophilic masses with eosinophilic terminal clubs on staining with hematoxylin and eosin.(19) Typical microscopic findings include necrosis with yellowish sulfur granules and filamentou
Gram-positive fungal-like pathogens. Yellowish sulfur granules are constituted by conglomeration of bacteria trapped in biofilm.(29) These findings are highly suggestive of the diagnosis but are not specific, as they can be encountered in other pathogenic conditions such as nocardiosis or chronic cervicofacial fungal infections. However, Gram staining can additionally show Gram-positive filamentous branching bacteria at the periphery of the granule that is highly suggestive of actinomycosis.
Immunofluorescence techniques have poor sensitivity but are highly specific in the diagnosis.
CONCLUSION
Actinomycosis is a rare chronic disease caused by Actinomyces spp., anaerobic Gram-positive bacteria that normally colonize the human mouth and digestive and genital tracts. Physicians have to be aware of typical clinical presentations (such as cervicofacial actinomycosis following dental focus of infection), but also must be aware that actinomycosis may mimic the malignancy process in various anatomical sites. Bacterial cultures and pathology are the cornerstones of diagnosis and require particular attention to prevent misdiagnosis. Prolonged bacterial cultures in anaerobic conditions are necessary for identification of the bacterium, and typical microscopic findings include necrosis with yellowish sulfur granules and filamentous Gram-positive fungal-like pathogens. Patients with actinomycosis require prolonged (6- to 12-month) high doses of penicillin G or amoxicillin, but the duration of antimicrobial therapy could likely be reduced (3 months) for patients in whom optimal surgical resection of infected tissues has been performed. Specific preventive measures (reduction of alcohol abuse, dental hygiene) may limit the occurrence of actinomycosis.
REFERENCES
-
- Wong VK, Turmezei TD, Weston VC. Actinomycosis. BMJ. 2011;343:d6099
- Schaal KP, Lee HJ. Actinomycete infections in humans – a review. Gene. 1992;115(1–2):201–211.
- Smego RA Jr, Foglia G. Actinomycosis. Clin Infect Dis. 1998;26(6): 1255–1261.
- Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, PA: Churchill Livingstone Elsevier; 2010.
- Pulverer G, Schütt-Gerowitt H, Schaal KP. Human cervicofacial actinomycoses: microbiological data for 1997 cases. Clin Infect Dis. 2003;37(4):490–497.
- Rajendran R. Shafer’s textbook of oral pathology. Elsevier India; 2009.
- Sullivan DC, Chapman SW. Bacteria that masquerade as fungi: actinomycosis/nocardia. Proceedings of the American Thoracic Society. 2010 May 15;7(3):216-21.
- Sezer B, Akdeniz BG, Günbay S, Hilmioğlu-Polat S, Başdemir G. Actinomycosis osteomyelitis of the jaws: report of four cases and a review of the literature. Journal of Dental Sciences. 2017 Sep 1;12(3):301-7.
- Belmont MJ, Behar PM, Wax MK. Atypical presentations of actinomycosis. Head & neck. 1999 May 1;21(3): 264-8.
- Crossman T, Herold J. Actinomycosis of the maxilla–a case report of a rare oral infection presenting in general dental practice. British dental journal. 2009 Feb 28;206(4):201-2.
- Bala S, Narwal A, Gupta V, Duhan J, Goel P. Actinomycotic osteomyelitis of mandible masquerading periapical pathology. J Oral Health Comm Dent. 2011;5(2):97-.
- Freeman LR, Zimmermann EE, Ferrillo PJ. Conservative treatment of periapical actinomycosis. Oral Surgery, Oral Medicine, Oral Pathology. 1981 Feb 1;51(2):205-8.
- Stenhouse D. Intraoral actinomycosis: Report of five cases. Oral Surgery, Oral Medicine, Oral Pathology. 1975 Apr 1;39(4):547-52.
- Strassburg M, Knolle G. Diseases of the Oral Mucosa–A Color Atlas. American Journal of Orthodontics and Dentofacial Orthopedics. 1994;106(2):222.
- Sadeghi EM, Hopper TL. Actinomycosis involving a mandibular odontoma. The Journal of the American Dental Association. 1983 Sep 1;107(3):434-7.
- Nagler RM, Ben-Arieh Y, Laufer D. Case report of regional alveolar bone actinomycosis: a juvenile periodontitislike lesion. Journal of periodontology. 2000 May 1;71(5):825-9.
- Felz MW, Smith MR. Disseminated actinomycosis: multisystem mimicry in primary care. South Med J. 2003;96(3):294–299.
- Holm P. Studies on the aetiology of human actinomycosis. II. Do the other microbes of actinomycosis possess virulence? Acta Pathol Microbiol Scand. 1951;28(4):391–406.
- Bennhoff DF. Actinomycosis: diagnostic and therapeutic considerations and a review of 32 cases. Laryngoscope. 1984;94(9):1198–1217.
- Lewis R, McKenzie D, Bagg J, Dickie A. Experience with a novel selective medium for isolation of Actinomyces spp. from medical and dental specimens. J Clin Microbiol. 1995;33(6):1613–1616.
- Ng LS, Sim JH, Eng LC, Menon S, Tan TY. Comparison of phenotypic methods and matrix-assisted laser desorption ionisation time-of-flight mass spectrometry for the identification of aero-tolerant Actinomyces spp. isolated from soft-tissue infections. Eur J Clin Microbiol Infect Dis. 2012;31(8):1749–1752.
- Miller PH, Wiggs LS, Miller JM. Evaluation of API An-IDENT and RapID ANA II systems for identification of Actinomyces species from clinical specimens. J Clin Microbiol. 1995;33(2):329–330.
- Garner O, Mochon A, Branda J, et al. Multi-centre evaluation of mass spectrometric identification of anaerobic bacteria using the VITEK®MS system. Clin Microbiol Infect. Epub July 4, 2013.
- Hall V, Talbot PR, Stubbs SL, Duerden BI. Identification of clinical isolates of Actinomyces species by amplified 16S ribosomal DNA restriction analysis. J Clin Microbiol. 2001;39(10):3555–3562.
- Brown JR. Human actinomycosis. A study of 181 subjects. Hum Pathol. 1973;4(3):319–330.
- Mabeza GF, Macfarlane J. Pulmonary actinomycosis. Eur Respir J. 2003;21(3):545–551.
- Pine L. Recent developments on the nature of the anaerobic actinomycetes. Ann Soc Belg Med Trop (1920). 1963;43:247–257.
- Hotchi M, Schwarz J. Characterization of actinomycotic granules by architecture and staining methods. Arch Pathol. 1972;93(5):392–400.
- Heffner JE. Pleuropulmonary manifestations of actinomycosis and noardiosis. Semin Respir Infect. 1988;3:352–361.
© Copyright 2023, All Rights Reserved. Use of this content signifies your agreement to the T&Cs of Unified Citation Journals
This abstract of Manuscript/Paper/Article is an open access Manuscript/Paper/Article distributed under the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/) which allows and permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited and accepted.
This communication and any documents, or files, attached to it, constitute an electronic communication within the scope of the Electronic Communication Privacy Act (https://it.ojp.gov/PrivacyLiberty/authorities/statutes/1285)
To citation of this article: Dr M Naveen Kumar 1, Dr. Bhargavi 2, Dr. Vinila Belum Reddy 3, Dr S Jayabhaskar Reddy 4, Dr Manimekhala P 5, Dr Vanajakshi S 6, Dr Bala Krishna 7, Studying The Individual Role And Interrelationship Between Mean Platelet Volume And Platelet Distribution Width In Predicting The Fall/Rise Of Platelet Count In Dengue, Global Journal of Pathology & Laboratory Medicine