Correlation of The Efficacy Of Convalscent Plasma In Covid – 19 Patients with The Titre Of IgG Antibodies In A Tertiary Care Setup
Global Journal of Pathology & Laboratory Medicine
Volume 1, Issue 4, January 2022, Pages: 51-58
Received: November 20, 2021, Reviewed: December 04, 2021, Accepted: December 11, 2021, Published: February 3rd, 2022
Unified Citation Journals, Pathology 2022, 1(4) 1-08; https://doi.org/10.52402/Pathology211
ISSN 2754-0952
Authors: Dr. Shubhangi Gupta 1, Dr. Manjit Singh Bindra 2, Dr. Gladys Rai 3, Dr. Shirish Pandey 4
Corresponding Author: Dr. Atul Verma
1 Assistant Professor, Dept. of Pathology, SMS&R, Sharda University, Greater Noida
2 Professor, Dept. of Pathology, SMS&R, Sharda University, Greater Noida
3 Professor & Head Dept. of Biochemistry, SMS&R, Sharda University, Greater Noida
4 Assistant Professor, Dept. of Medicine, SMS&R, Sharda University, Greater Noida
Corresponding Author: Assistant Professor, School of Medical Sciences and Research
Download PDFIntroduction
Severe Respiratory Syndrome– Coronavirus 2 (SARS-CoV2) or Coronavirus disease2019 (Covid -19) is presently estimated to have infected more than 3.5 million individuals in India and more than 30 million worldwide. The Covid -19 is a great threat to the communities worldwide. 1
Currently, there are no approved treatments for COVID-19. The management plan is supportive care with supplemental oxygen and mechanical ventilation2. Multiple trials are being done across the globe to assess the efficacy of various treatment strategies. WHO initiated the SOLIDARITY trial in several countries to compare the effectiveness of the following regimens against COVID-19: Remdesivir, Lopinavir/Ritonavir, Lopinavir/Ritonavir with interferon beta, and hydroxychloroquine3. Since the effective vaccine and specific antiviral medicines are under trial, Convalescent plasma (CP) therapy, a classic for Covid-19 by Govt. of India adaptive immunotherapy, has been applied to the prevention and treatment for COVID-19 in the Clinical Management Protocol4. Over the past two decades, CP therapy was successfully used in the treatment of SARS, MERS, and the 2009 H1N1 pandemic with satisfactory efficacy and safety 5-9. US FDA has also recently approved Convalescent Plasma from patients who recovered from COVID 19 for the treatment of severe or life-threatening COVID-19 infections10.
Convalescent Plasma (CP) Therapy includes the administration of immunoglobulins containing plasma of a recently recovered individual from a specific infection (SARS-2) to any individual who is susceptible or, infected (has manifested symptoms) with COVID-19) for the purpose of prophylaxis and treatment 11. Immunized plasma acts by binding to a given pathogen including virus (SARS-2) directly and causing its denaturalization, eventually, eradicating the latter from the peripheral bloodstream while other antibody-mediated pathways including complement system, antibody-dependent cell-mediated cytotoxicity, and phagocytosis might, also, contribute towards the therapeutic effects achieved 12. Currently, there are several studies summarizing the results of CP transfusion in COVID-19 to be safe and effective.
In the present study, we focused on assessing the usefulness of transfusing convalescent plasma based on the titre of IgG antibodies present in COVID-19 patients.
Methodology :
Aim and objectives
- To record the clinical findings and classify the disease severity in prospective plasma recipients before transfusion of convalescent plasma.
- To record the clinical findings and classify the disease severity in patients after transfusion of convalescent plasma.
- To correlate the titre of IgG antibodies with change in disease severity if any based on clinical findings in COVID -19 patients after receiving convalescent plasma therapy.
Table 2
Materials and methods
Our study was a Retrospective and Prospective Analytical study done at the Department of Blood bank, Central lab, COVID ward and ICU, Sharda Hospital, Greater Noida from July 2020 to June 2021. The selection criteria for cases for Plasma recipients were SARS COV 2 positive patients already confirmed by RT-PCR Test before admission and who are Moderately sick COVID-19 patients in whom Convalescent plasma therapy is indicated as per the ICMR guidelines. Exclusion Criteria for Plasma recipients were Asymptomatic, mildly symptomatic, and severely symptomatic patients with a history of a major transfusion reaction in the past.
For the retrospective cases, the medical records were screened to identify patients who have received plasma therapy at Sharda Hospital as per selection criteria. Detailed history and relevant general and systemic clinical examination of subjects were recorded from the case history sheets of enrolled patients who were admitted to the COVID Isolation, COVID wards, and COVID ICU of Medicine department. A total of 205 COVID-19 patients were recruited who underwent convalescent plasma transfusion. For determining the Anti- SARS-CoV-2 IgG titre, VITROS Immunodiagnostic Kit was used along with its Calibrator for the qualitative detection of IgG antibodies. As per the literature provided with the kit, tests were found to have a signal-to-cutoff (S/C) value of 12 or greater qualify as a high titer. The study was performed as per the ethical guidelines established and was approved by the institutional ethics committee.
Statistical analysis
- All statistical data will be coded, entered, and analyzed by using the freely available version of SPSS.
- All the data will be expressed as mean ± SD /SEM.
- The difference between the variables will be evaluated using the student’s test.
- The p-value of ≤ 0.05 will be considered significant.
RESULTS:
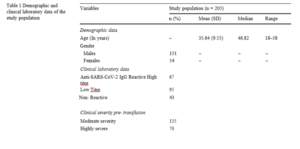
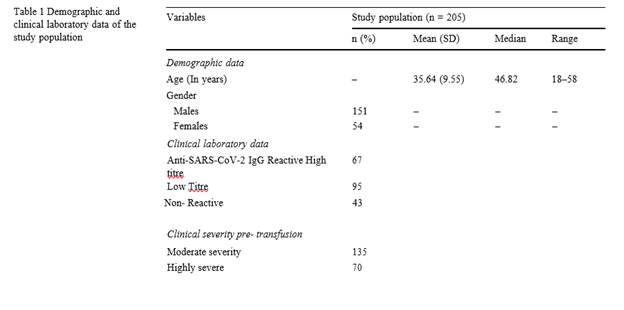
The demographic profiles, biochemical profile (Anti-SARS-CoV-2 IgG antibody titre, total protein, albumin, and globulin), clinical symptom profiles (throat pain, cough, fever, dyspnoea, muscle/ body pain, and loss of taste/smell), complete blood count were recorded (Tables 1).
The mean (SD) age of the study population was 35.6 (9.3) years while the median and range were 36 and 42- 58 years respectively. Among the study subjects, 151 (73.6%) were males with a mean age of 46.2 years and 54 (26.4%) were females with a mean age of 38.1 years.
In the total study population, 83 (40%) were having O positive blood group, 69 (3%) were having B positive blood group, 45 (21%) were having A positive blood group & 13 (6%) were having AB positive blood group. Out of all the positively tested individuals (145) for antibodies, 57 (39.3%) had O positive blood group. On comparing the blood groups with the antibody status and other clinical parameters, no significant difference was found.
Out of the total study population, the on analyzing the symptoms, the maximum number of study subjects were having throat pain (85.7%), fever (73.8%) and cough (68%). Out of the 205, plasma transfused patients, the clinical severity was moderate in 135 cases, while 70 were severe. The antibody titer of IgG was evaluated in the patients post-transfusion of convalescent plasma in these patients, to see the rise in titre of antibodies, hypothesizing that these patients were not vaccinated, hence, there were no preformed antibodies in them. The titer of IgG antibodies was found to be High in 67 cases, whereas a low titre was observed in 95 cases, and in 43 cases, the titer was non-reactive. On comparing the serum anti-SARS-CoV-2 IgG anti-body titre between individuals with post-transfusion, a significant increase in the antibody levels was observed among the subjects having cough (p = 0.0004). Logistic regression was run to understand the effects of the clinical features and symptoms on the development of antibodies. On correlation analysis, no significant correlation was found between Anti-SARS-CoV-2 IgG antibody titres and biochemical parameters (Table1).
We assessed the correlation with the change in the severity of the patients. Post transfusion, out of the 67 patients with a high titre, 32 cases showed an improvement in the clinical condition, 28 deteriorated and 7 died. Out of the 95 cases with a low titre, the clinical condition improved in 51 % of cases, whereas the rest had deteriorated condition. Out of the 43 patients showing non–reactive titer, there was a clinical improvement seen in 27 cases.
DISCUSSION:
Ever since WHO declared COVID-19 as a global pandemic, the whole world has been looking for the development of an effective treatment or vaccine against SARS- CoV-2 infection. The COVID-19 pandemic has become a major public health challenge around the world. Recent studies have reported the use of convalescent plasma may be an effective and safe treatment option to help control the COVID-19 pandemic. There are now thousands of recovered COVID-19 patients who could donate convalescent plasma. Establishing optimal efficacy and titres for COVID-19 convalescent plasma donors is necessary to ensure convalescent plasma therapeutic potency and to optimize collection efficiency13-14.
Convalescent plasma has been tried to fight pathogens including Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV), Ebola virus, and the Middle East Respiratory Syndrome Coronavirus (MERS-CoV), and avian influenza A (H5N1) virus.9 The recent COVID-19 outbreak worldwide has prompted the exploratory use of convalescent plasma in treating COVID-19, and case reports and case series have shown encouraging results11.
SARS-CoV-2 is an enveloped virus with four structural proteins: spike (S) protein, membrane (M) protein, enveloped (E) protein, and nucleocapsid (N) protein. The S protein receptor-binding domain (RBD) has been identified as a key target for therapeutic antibodies, as it plays essential roles in tropism and virus entry into host cells and can induce neutralizing antibodies and protective immunity. A recent study by Walls et al12 found that SARS-CoV S-murine polyclonal antibodies potently inhibit SARS-CoV S mediated entry into cells and induce the production of protective neutralizing antibodies targeting S epitopes. Thus, various studies predict that S-RBD-specific IgG may be a protective antibody and that its level would significantly increase in recovered patients. Cao et al.14 studied SARS-CoV neutralizing antibody titers in 56 patients who recovered from SARS-CoV infections. Their findings show that SARS-CoV IgG and neutralizing antibodies peaked at 4 months and then declined, reaching undetectable levels in 25.6% (IgG) and 16.1% (neutralizing antibodies) of study subjects at 36 months. Ideally, COVID-19 convalescent plasma products should be measured with viral neutralization antibody titer to ensure product potency.. Using S-RBD-specific IgG titer as a surrogate marker for viral neutralization titer is far more practical and brings convalescent plasma product quality control for potency a step closer to reality10.
Various studies relating to the titer of antibodies – a test that measures the quantity and variety of antibodies is dependent on the initial viral load and disease severity. Antigen burden plays a key role in elucidating the magnitude of the immune response. It has been observed that the highest titers of neutralizing antibodies were noted in recovered patients with severe disease. Those with mild/asymptomatic disease showed low titres and low viral neutralizing activity against the RBD of the viral S protein16.
According to the Clinical management Protocol designed by the Health Ministry, Govt of India currently Convalescent plasma therapy is being considered as an investigational therapy in patients with the moderate disease who are not improving (oxygen requirement is progressively increasing) despite the use of steroids. Neutralizing titer of donor plasma should be above the specific threshold (if the latter is not available, plasma IgG titer (against S-protein RBD) above 1:640 is being used). The dose is variable ranging from 4 to 13 ml/kg (usually 200 ml single dose given slowly over not less than 2 hours1.
Currently, there is insufficient data about how titer levels correlate with viral clearance and even the roles of humoral and cellular immune responses during the infection. In this study, we made a database of potential donors for convalescent plasma therapy by assessing the Anti-SARS-CoV-2 IgG levels in treated and recovered patients from COVID-19.
Quantification of Anti-SARS-CoV-2 IgG antibody levels in recovered or treated patients is a key tool for the determination of immunity of the population to SARS- CoV-2 infection. Results of our study suggest that majority of the subjects who have recovered from SARS-CoV-2 infection have high titre. Although most of the study subjects have a similar duration of hospital stay, the serum antibody titres showed significant variation. Around 67% of the study population failed to develop a significant antibody titre against the SARS- CoV-2. This may be due to different immune responses in individuals against COVID-19 around the globe. Similarly, few individuals had developed very high titres of Anti- SARS-CoV-2 IgG levels within 20 days with the same course of infection as individuals who failed to develop antibodies. These findings were similar to the study done by Wu et al., where 30% of the study population who had recovered from SARS-CoV-2 infection failed to develop the antibodies against the virus18. These results may suggest that some other immune factors may play role in the development of the Anti-SARS-CoV-2 IgG in recovered patients. Whether these individuals with low antibody titres are at risk of reinfection has to be explored in future studies.
Like other coronavirus infections, in SARS-CoV-2 infection, age played a major role in severity. In a recent study by Yang et al., patients aged 19–30 years exhibited lower levels of SARS-CoV-2 IgG antibodies than children and older adults 19. However, in this study, middle-aged and old people had high Ab titres compared to younger individuals. The exact mechanisms underlying the different SARS-CoV-2 immune responses based on age remain unclear. Age-related quantitative and qualitative changes in the immune system affect cells and soluble mediators of both the innate and adaptive immune responses within lymphoid and non-lymphoid peripheral tissues.
CONCLUSION:
Among the patients who recovered from SARS-CoV-2 infection, Anti-SARS-CoV-2 IgG antibody titres appeared to vary substantially. The development of the antibodies positively correlated with age and clinical severity. Further, the data suggest that blood groups may have a lesser impact on the severity and the development of the antibodies. The potential clinical implications of these findings for convalescent plasma therapy, vaccine development and future protection from infection are still needed to be explored further.
This study is preliminary and has several limitations. First, the viral loads during the disease course have not been taken into consideration. Second, we did not measure the neutralising antibodies hence we were unable to evaluate the effect of viral clearance on antibodies. Additionally, the treatment was an admixture of multiple therapies, rather than convalescent plasma transfusion alone. Hence, the efficacy of plasma transfusion as an effective treatment option cannot be deduced conclusively.
References:
[1] Revised Guidelines on clinical management of COVID-19. Government of India, Ministry of Health & Family Welfare, Directorate General of Health Services (EMR Division) March 2020: 2-3
[2] World Health Organization (WHO). Coronavirus disease (COVID-19) pandemic. [Accessed 10 Jun 2020]. Geneva: WHO. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019
[3] An EU programme of COVID-19 convalescent plasma collection and transfusion Guidance on collection, testing, processing, storage, distribution and monitored use. [Accessed 12 Jun 2020]. Available from: https://ec.europa.eu/health/sites/health/files/blood_tissues_organs/docs/guidance_plasma_covid19_en.pdf
[4] Recommendations for Investigations COVID-19 Convalescent Plasma recommendation. 1 May 2020. [Accessed 12 Jun 2020]. Available from: https://www.fda.gov/vaccines-blood-biologics/ investigational-new-drug-ind-or-device-exemption-ideprocess- cber/recommendations-investigational-covid-19-convalescent-plasma
[5] Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci USA. 2020;117(17):9490-6. https://doi.org/10.1073/pnas.2004168117 PMID: 32253318 .
[6] Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J,et al. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA. 2020;323(16):1582-9. https://doi.org/10.1001/jama.2020.4783 PMID: 32219428
[7] Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novelcoronavirus in Wuhan, China. Lancet 2020;395(10223):497–506.
[8]Worldmeter Corona cases: https://www.worldometers.info/coronavirus/ (access date August 8th 2020)
[9] Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 2020;382(19):1787–99.
[10] Maor et al. / EClinicalMedicine 26 (2020) 100525
[11] Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Napoli RD, et.al. (2020) Features, Evaluation and Treatment of Corona virus (COVID-19). StatPearls [Internet]. Treasure Island (FL): Stat Pearls Publishing.
[12] Walls AC, Park YJ, Tortorici MA, et al. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020;181:281-92.
[13] New RRC, Moore BD, Butcher W, et al. Antibody-mediated protection against MERS-CoV in the murine model. Vaccine 2019;37:4094-102.
[14] Cao WC, Liu W, Zhang PH, et al. Disappearance of antibodies to SARS-associated coronavirus after recovery. N Engl J Med 2007;357:1162-3.
[15] Zhao J, Yang Y, Huang H-P, et al. Relationship between the ABO blood group and the COVID-19 susceptibility. medRxiv 2020. (Published article online). https://doi.org/10.1101/2020.03.11.20031096
[16] https://epaper.hindustantimes.com/Home/MShareArticle?OrgId=2296c09571d
[17] Garcia-Basteiro AL, Moncunill G, Tortajada M, Vidal M, Guinovart C, Jime´nez A, et Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nat Commun [Internet]. 2020 [cited 2020 Dec 28];11. Available from: https://www.ncbi.nlm.nih.gov/ pmc/articles/PMC7343863
[18] Liu J, Liu Y, Xiang P, Pu L, Xiong H, Li C, et al. Neutrophil-to- lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early J Transl Med. 2020;18(1):206.
[19] Zhang B, Zhou X, Zhu C, Song Y, Feng F, Qiu Y, et al. Immune Phenotyping Based on the neutrophil-to-lymphocyte ratio and IgG level predicts disease severity and outcome for patients with COVID-19. Front Mol Biosci [Internet]. 2020 [cited 2020 Dec 28];7. Available from: https://www.frontiersin.org/articles. Doi: https://doi.org/10.3389/fmolb.2020.00157/full#B6
[20] Peiris JSM, Chu CM, Cheng VCC, Chan KS, Hung IFN, Poon LLM, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective Lancet Lond Engl. 2003;361(9371):1767–72.
[21] Hong K-H, Choi J-P, Hong S-H, Lee J, Kwon J-S, Kim S-M, et al. Predictors of mortality in the Middle East respiratory syndrome (MERS). 2018;73(3):286–9.
© Copyright 2022, All Rights Reserved. Use of this content signifies your agreement to the T&Cs of Unified Citation Journals
This abstract of Manuscript/Paper/Article is an open access Manuscript/Paper/Article distributed under the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/) which allows and permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited and accepted.
This communication and any documents, or files, attached to it, constitute an electronic communication within the scope of the Electronic Communication Privacy Act (https://it.ojp.gov/PrivacyLiberty/authorities/statutes/1285)
To citation of this article: Dr. Shubhangi Gupta, Dr. Manjit Singh Bindra, Dr. Gladys Rai, Dr. Shirish Pandey.
Corresponding Author: Assistant Professor, School of Medical Sciences and Research
Correlation of The Efficacy Of Convalscent Plasma In Covid – 19 Patients with The Titre Of IgG Antibodies In A Tertiary Care Setup, Global Journal of Pathology & Laboratory Medicine