Telehealth and Virtual Clinical Trials: The Interplay of Data Engineering and Cloud Technologies for a Digital-First Healthcare Approach Use
Deepak Singh, Sreeram M, Sanju Mannumadam Venugopal, Suyash Bhogawar
Unified Nursing Research, Midwifery & Women’s Health Journal
Volume 2, Issue 1, August 2023
Unified Citation Journals, Nursing, Healthcare 2023, 2(1) 16-25; https://doi.org/10.52402/Nursing2014
ISSN 2754-0944
Authors: Deepak Singh 1, Sreeram M 2, Sanju Mannumadam Venugopal 3, Suyash Bhogawar 4
1 Product Manager, Telehealth Firm, CA, USA,
2 Senior Product Manager, Remote Patient Management Firm, MA, USA
3 Senior Manager, Health Tech Firm, CA, USA
4 Data Science Solution Architect, Cloud Technology Firm, CA USA
Category: Original research
Country: USA
Address: CA, USA
Journals Short Code: UNRMWHJ
ORCHID: 0009-0000-0952-2604
Keywords: Telehealth, Virtual Clinical Trials, Cloud Technologies, and Digital-First Healthcare Approach
Abstract: The term digital health describes processes like telemedicine and mobile health that supply healthcare resources, services, and information through electronic technologies. In this review of the literature, previous studies on the effects of telehealth and virtual clinical trials on healthcare are identified and analyzed. The review of the literature also looks at the interactions between cloud technologies and data engineering when applied to a digital-first healthcare strategy. This research design made use of a systematic review. PRISMA was the search engine used for this study. Articles published between 2010 and 2023 were used in this investigation. After applying the exclusion criteria, 11 studies were discovered and assessed using the CASP checklist. The part that virtual visits and remote monitoring can play in situations where receiving treatment in a facility is difficult. Less scheduled outpatient visits were necessary for high-risk obstetrics because of the patient-generated data that was communicated via remote monitoring and smartphones. The telemedicine service was as safe and efficient as receiving care in person. Future research should look at how these interventions might be used to provide and manage contraception, among other services that some patients might find challenging to get.
Introduction: The healthcare sector has found it particularly challenging to make the shift to digital technology because of the growing demand for services brought on by the ageing population and the emergence of new ailments [1]. Investment in novel cures is thus necessary to guarantee that everyone has the same opportunity to the healthcare system [2]. Digital health refers to practices like telemedicine and mobile health that supply healthcare resources, services, and information through electronic technologies [3]. Mobile devices are used in mobile health, also known as mhealth, to help patients manage or monitor their treatments, difficulties, or other health-related issues [4]. Patients can also utilise applications to check information and request services electronically [5].
1.1 The digital shift in the healthcare industry The concept of “digital health,” also referred to as “digital healthcare,” is broad and diverse and includes concepts from the intersection of technology and healthcare [5]. Through the usage of digital health, software, technology, and services are all integrated into the healthcare sector [6]. The phrase “digital health” encompasses a wide range of practices, including wearable technology, telehealth and telemedicine, customized healthcare, electronic health records (EHRs), electronic medical records (EMRs), and applications for mobile health (mHealth) [7]. Stakeholders in the field of digital health include patients, medical professionals, and studies, developers of applications, and manufacturers and distributors of medical equipment. In the contemporary healthcare system, the value of digital healthcare is growing [8].
1.1.1 Telehealth/Digital Healthcare Telehealth is defined as the provision of healthcare services by any healthcare provider using telecommunications and information technology for the exchange of reliable information for illness diagnosis, treatment, and prevention where distance is an issue [9]. A live video conference or phone chat with a medical professional is part of synchronous telehealth care. AI-powered digital personal assistants are one example of a technology that has the potential to be used as medical devices for monitoring, assistance, and advising. These technologies serve as a bridge between the public and healthcare systems, impacting decision-making across a range of industries, including healthcare [10]. Email, text messaging, and patient portals are examples of asynchronous telehealth communication methods [11]. Receiving vital signs and pictures from a patient for remote monitoring enables diagnosis and treatment for a particular health issue [12].
1.1.2 Clinical Trials in a virtual setting Decentralized clinical trials (DCTs), also referred to as virtual clinical trials (VCTs), use technological innovations and other approaches that differ from traditional trial models to bring research closer to patients’ homes and improve access to trials [13] while minimizing inconvenience and burden on participants by lowering the number of physical site visits [14]. Trials conducted virtually are frequently referred to as siteless, electric or hybrid or digital trials. Digital biomarker collection, wearables, remote patient monitoring, telemedicine, virtual clinical outcome assessment, and eConsent are a few examples of digital technology utilized in virtual studies [15].
During the COVID-19 pandemic, telemedicine proved to be a crucial tool for connecting patients with healthcare professionals and providing care while ensuring their safety and lowering the danger of transmission [16]. As a result, it was found that telemedicine was an essential strategy used to decrease the impact of the COVID-19 pandemic on clinical trial disruptions [17].
Advanced analytics and insight generation, interoperability between different eClinical software systems, secure single accessibility for study participants [9], and the dependable and secure collection and coordination of enormous amounts of data from numerous disparate sources are all supported by cloud-based virtual trial platforms. Additionally, it enables the easy scaling up of research using a variety of methods to serve a large number of patients [18]. VR technology is now more accessible, adaptable, and portable than ever before, making it useful for therapeutic use in both inpatient and outpatient settings. Display devices are essential for VR development because interactive VR systems use PCs, gaming consoles, or cellphones for real-time animation, positioning tracking, and audio-visual simulation. [19].
1.2 Purpose of the Study
Digital transformation has altered potential subjects’ perspectives in addition to researchers’ methods [15]. By reducing in-person visits and direct communication between doctors and patients, the adoption of virtual healthcare services can help reduce the transmission of the virus and protect medical workers from infection [20], [21], [23]. The impact of Telehealth and Virtual Clinical Trials on healthcare is examined in this review of the literature. The literature evaluation also examines how cloud technologies and data engineering interact when used in a digital-first healthcare approach. A certain research question is examined into:
- What are the effects of remote patient management and telehealth?
- How do cloud technologies affect virtual clinical trials and what are their roles?
- What are the key uses of data engineering in virtual clinical trials and telehealth?
1.3 Importance of the study
Clinical studies that incorporate digital technology, such as telemedicine, are known as decentralized clinical trials. Decentralized trials can enroll participants from different geographic locations by utilizing digital technology, which has the effect of making these studies widely accessible. Decentralized clinical trials frequently incorporate some type of remote patient monitoring that enables researchers to track the progress of study participants in real time and get information directly from patients.
2.2 Data collection and extraction Online publications and browsers were employed for the data collection, and the majority of them produced high impact, peer-reviewed articles. The data was gathered using the search engines ScienceDirect, PUBMED, and Google Scholar.
2.3 Inclusion criteria and Exclusion criteria
- The language of the articles is English.
- In light of the study’s findings, how may telehealth and virtual clinical trials be used in the context of data engineering and cloud technologies for a digital-first healthcare approach?
- Published year (from 2010 through 2023).
- A study on healthcare’s use of virtual clinical trials and telehealth.
- Articles and proceedings are considered to be publications of sufficient quality.
- Articles that provide less information regarding virtual clinical trials and telehealth
- Before 2010 8. Translation errors caused papers that weren’t published in English to be left out.
- Articles that omitted the full text of material relevant to the review’s subject.
Method
The current study used the mixed-methods systematic review methodology. PRISMA approaches for data extraction were used.
2.1 Design The mode, orientation, and structure of the research all influence how the work is designed. The design makes it easier for the researcher to gather the greatest information and answers in relation to the study questions. As a result, the design of the current work was an analysis of the system using qualitative research techniques.
2.2 Data collection and extraction Online publications and browsers were employed for the data collection, and the majority of them produced high impact, peer-reviewed articles. The data was gathered using the search engines ScienceDirect, PUBMED, and Google Scholar.
2.3 Inclusion criteria and Exclusion criteria
- The language of the articles is English.
- In light of the study’s findings, how may telehealth and virtual clinical trials be used in the context of data engineering and cloud technologies for a digital-first healthcare approach?
- Published year (from 2010 through 2023).
- A study on healthcare’s use of virtual clinical trials and telehealth.
- Articles and proceedings are considered to be publications of sufficient quality.
- Articles that provide less information regarding virtual clinical trials and telehealth
- Before 2010 8. Translation errors caused papers that weren’t published in English to be left out.
- Articles that omitted the full text of material relevant to the review’s subject.4 Quality AppraisalThe research’s validity was evaluated, the work’s analytical quality was examined, and the biases in some of the data were minimised using the critical appraisal method. The critical appraisal skills programme (CASP) is the name of the specific tool used to collect data in relation to the study topics. All of the research that were included in the final theme synthesis underwent an analysis using the CASP technique, and they were only finished after it had been validated.
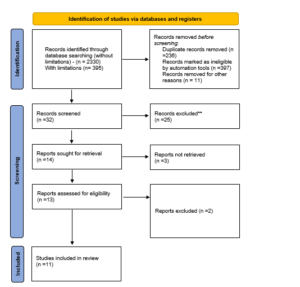
Preferred reporting items for systematic reviews and meta-analysis (PRISMA) was the search technique utilized for data extraction, scrutiny, exclusion, inclusion of the publications, and finalization of the research used for the results and discussion section. Utilizing abstract analysis, article titles, and keyword analysis, the most relevant articles that may be considered for the thematic analysis were identified. A review of the abstracts led to the selection of 32 papers for the thorough evaluation. The possibility of discussing full articles in the conclusion was looked at. When reading the abstract, keywords, introduction, methodology, results, and discussion sections as well as the conclusion, it is important to keep the search objectives in mind. Following this stage, 13 articles were
further eliminated from contention for the final selection because they fell short of meeting all of the inclusion and exclusion criteria that had previously been synthesized. Eleven publications in all were used for the overall systematic theme meta-analysis.
Figure 1: PRISMA Diagram show eleven studies were located, as shown in the data extraction findings in the above table. Every piece should be subjected to a topic or content examination.
Result
3.1 PRISMA The study selection procedure led to the discovery of 395 original papers. The thorough review, elimination, and article selection processes are depicted in Figure 1. After being evaluated for their titles or abstracts, fourteen papers were disregarded, and full-text analyses were done on 32 publications. 11 studies could be found after the exclusion criteria and evaluated for quality. One study was excluded due to a lack of identifying information. The complete synthesis of key concerns, which fully satisfied the predefined outcomes of the current systematic review, has already been mentioned in part. Clinical Trials in a Virtual Environment, Remote Patient Management in Healthcare, Use of Data Engineering and Cloud Technologies in Healthcare, and other relevant issues have all been selected as essential topics that correlate with the current systematic review’s predetermined objectives.
The study selection procedure led to the discovery of 395 original papers. The thorough review, elimination, and article selection processes are depicted in Figure 1. After being evaluated for their titles or abstracts, fourteen papers were disregarded, and full-text analyses were done on 32 publications. 11 studies could be found after the exclusion criteria and evaluated for quality. One study was excluded due to a lack of identifying information. The complete synthesis of key concerns, which fully satisfied the predefined outcomes of the current systematic review, has already been mentioned in part. Clinical Trials in a Virtual Environment, Remote Patient Management in Healthcare, Use of Data Engineering and Cloud Technologies in Healthcare, and other relevant issues have all been selected as essential topics that correlate with the current systematic review’s predetermined objectives.
3.2 Theme 1: Telehealth and Remote Patient Management It could be possible to get over some of barriers via remote patient management (RPM) [25]. RPM, which extends interactive interaction from traditional clinical venues to the patient’s home [26], is a framework for remotely monitoring patients. RPM offers a chance to enhance patient outcomes while also possibly lowering the cost of the associated medical care [27]. Given that PD is often less expensive to offer than in-center hemodialysis, these cost savings could be attained by increasing the uptake of PD patients and decreasing the rate of PD technique failure [1].
The initial and ongoing expenditures of the technology may potentially prevent RPM programs from being implemented [28]. The labor involved in implementing an RPM program is a cost. In particular, RPM can demand a large amount of nursing time to analyze data if it is not incorporated into the patient’s usual care and if it is not used efficiently [2]. In addition, telehealth implementation laws, such as those pertaining to information security and payment standards, have slowed the adoption of telehealth technology in nephrology [29].
3.3 Theme 2: Virtual Clinical Trials and the Role of Cloud Technologies Clinical trials are conducted to evaluate the effectiveness and security of new medical technologies or treatments. Despite the significant contribution that clinical trials make to the development of medicine [2], access to the majority of clinical trial data has been severely constrained. Data sharing can improve clinical care and lead to new developments in the administration of clinical trials, tailored treatment regimens, and improved modifications to the technologies or drugs now being tested [4].
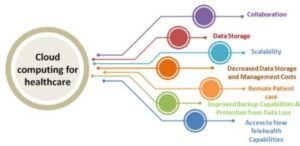
In clinical trials, the patient is chosen, qualified, randomized, and given the medication through the investigator [17]. Cost-effective and simple to set up, cloud computing has a number of other useful advantages in the healthcare industry [25]. By making data more available and interoperable [31], cloud-based services serve to strengthen the health infrastructure and offer crucial assistance [27]. The usage of cloud-based technologies in healthcare systems has a number of benefits. Some of the main benefits of these technologies are illustrated in Fig. 2 [4].
Figure 2: Benefits of these technologies
Even when clinical study settings have been extensively reviewed and authorized by ethical committees, releasing such data might nonetheless lead to discussions regarding them [29]. Other investigators claiming bogus ownership of discoveries from clinical trials or flawed secondary analyses are some additional problems of data sharing, in addition to the fundamental concern of safeguarding patient privacy [1]. The existing method for sharing clinical trial data that solves the issues outlined above frequently entails complex data request proposals and protracted approval processes, which impede exploratory data analysis [4].
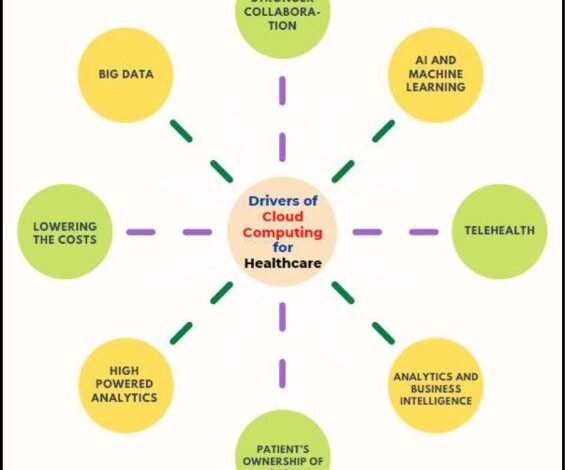
3.4 Theme 3: Data Engineering in Telehealth and Virtual Clinical Trials: The delivery of healthcare globally is now made easier by a number of technological innovations (electronic technologies that use mobile devices and/or the Internet) [25]. Digital tool development and use are expanding, providing novel approaches to healthcare [17]. Examples include wearable technology, telehealth, mobile health (mHealth), and other information technologies. Mobile technologies present a chance to collect outcomes assessed by mobile devices as well as to capture, store, manage, and transfer massive amounts of data [4]. Rosa et al.’s [3] illustration, however, shows that researchers are not frequently utilizing new methods to gather outcome data from clinical trials.
Figure 3: Important Cloud Computing Enablers for Healthcare [4].
Various mobile technologies are being creatively combined in other research to perform trials, or technology is being combined with conventional in-person methods for more hybrid designs [25], [1], and [28]. For instance, the Adaptable trial combined digital methods with conventional in-person visits for recruiting and data collection to examine the efficacy of two dosages of aspirin in preventing ischemic adverse events. Increased engagement by patients in their health plans and better patient outcomes by giving them access to their medical records [24].
Discussion:
Digital technologies have recently been utilized to evaluate study viability, make participant recruiting and retention easier, provide access to a variety of demographics, speed data collecting, and make data management easier [7]. Benefits include reduced costs, quick enrolment, and more thorough follow-up data, which increases the generalizability of outcomes [1]. On the other hand, some of the difficulties mentioned in our prior publication and others still exist [3]. Issues relating to data quality as well as ethical and privacy concerns are a few of the major obstacles [25].
As the digital divide continues to narrow, an advantage that is becoming more and more apparent is the possibility to enroll a more representative sample for studies [4]. According to data from 2018 [17], more people in rural areas and elderly people have access to the Internet via cellphones or broadband. The spread of digital tools (like blockchain and improvements in artificial intelligence) that address issues with data accuracy and participant privacy [23] and the development of more explicit suggestions from ethical and governmental groups on integrating digital technology approaches in the implementation of clinical trials [30][32] are probably the two most beneficial advances since the publication of our previous report five years ago [15].
Furthermore, the obstacles that must be overcome in order to provide care via telehealth could discourage even the most eager doctors from doing so. The possible liability associated with remote patient monitoring is one of these obstacles [2]. Critical vital indicators like dangerously low or high blood pressure will be recorded with remote monitoring [23]. Health care providers may be held liable if key values are not addressed on time, for instance because of provider weariness. To prevent some of these problems, consent documents for remote monitoring that outline the patient’s expectations of the program are required [28].
Comparatively to the patient management in healthcare, empirical research that take into account telehealth and virtual clinical trials are still in their infancy [19]. Numerous virtual clinical trials vendors have started to provide cutting-edge virtual clinical trials solutions as the next wave of health informatics/digital medical technologies are being deployed in the healthcare sector. The incorporation of Telehealth and Virtual Clinical Trials features is a relatively recent development, even if the use of digital tools in organisational and medical procedures in the healthcare industry is not new. Telehealth and virtual clinical trials still outperform people in terms of accuracy, efficiency, and rapidity of execution for healthcare and related administrative processes [19]. These developments could significantly affect not only the healthcare sector’s future but also the course of humanity [21]. The benefits for patients relate to clinical safeguarding, the experience of patients, and holistic care delivery; each of these aspects can be investigated from a clinical or psychological perspective [26].
Conclusion
The chosen literature reviewed in this paper serves as an illustration of how digital technology might improve specific areas of the design and execution of clinical trials. The part that virtual visits and remote monitoring can play in situations where receiving treatment in a facility is difficult. In the case of high-risk obstetrics, the management required fewer scheduled outpatient visits as a result of patient-generated data transmitted via remote monitoring and mobile phones. The telemedicine service was as safe and efficient as receiving care in person. Future research should look at how these interventions might be used to provide and manage contraception, among other services that some patients might find challenging to get.
5.1 Research Gaps and Future Directions There are several restrictions on this study. First, as academic papers frequently lack details about how Virtual Clinical Trials performs because these functionalities are typically proprietary in nature, there was a lack of particular Telehealth and Virtual Clinical Trials that could not be accessible. Second, despite the execution of a thorough search strategy, certain research on Virtual Clinical Trials in Healthcare was eliminated, such as grey literature and findings that have not been published in the selected databases that were examined.
Future research in this area could provide vital data for using the Digital-First Healthcare Strategy in clinical settings. In order to further strengthen the existing body of evidence for telehealth and virtual clinical trials, future reviews should consider evaluating the cost-effectiveness and results indicators that may affect the uptake and outcomes of telehealth efforts, such as healthcare provider satisfaction, patient medical outcomes (e.g., quality of life and care), as well as the usability and sustainability of self-monitored devices for diabetes management. These are all crucial pieces of data supporting current clinical recommendations and financial health-related legislation.
References
- L. Wallace et al., “Remote Patient Management for Home Dialysis Patients,” Kidney International Reports, vol. 2, no. 6, pp. 1009–1017, Nov. 2017, doi: https://doi.org/10.1016/j.ekir.2017.07.010.
- Luthria and Q. Wang, “Implementing a Cloud Based Method for Protected Clinical Trial Data Sharing,” Pacific Symposium on Biocomputing. Pacific Symposium on Biocomputing, vol. 25, pp. 647–658, 2020, Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6954005/
- Rosa, L. A. Marsch, E. L. Winstanley, M. Brunner, and A. N. C. Campbell, “Using digital technologies in clinical trials: Current and future applications,” Contemporary Clinical Trials, vol. 100, p. 106219, Jan. 2021, doi: https://doi.org/10.1016/j.cct.2020.106219.
- Javaid, A. Haleem, R. P. Singh, S. Rab, R. Suman, and I. H. Khan, “Evolutionary trends in progressive cloud computing based healthcare: Ideas, enablers, and barriers,” International Journal of Cognitive Computing in Engineering, vol. 3, pp. 124–135, Jun. 2022, doi: https://doi.org/10.1016/j.ijcce.2022.06.001.
- Sasangohar, E. Davis, B. A. Kash, and S. R. Shah, “Remote Patient Monitoring and Telemedicine in Neonatal and Pediatric Settings: Scoping Literature Review,” Journal of Medical Internet Research, vol. 20, no. 12, p. e295, Dec. 2018, doi: https://doi.org/10.2196/jmir.9403.
- Nakamura, T. Koga, and H. Iseki, “A meta-analysis of remote patient monitoring for chronic heart failure patients,” Journal of Telemedicine and Telecare, vol. 20, no. 1, pp. 11–17, Dec. 2013, doi: https://doi.org/10.1177/1357633×13517352.
- Raimo, I. De Turi, F. Albergo, and F. Vitolla, “The drivers of the digital transformation in the healthcare industry: An empirical analysis in Italian hospitals,” Technovation, p. 102558, May 2022, doi: https://doi.org/10.1016/j.technovation.2022.102558.
- Hermes, T. Riasanow, E. K. Clemons, M. Böhm, and H. Krcmar, “The digital transformation of the healthcare industry: exploring the rise of emerging platform ecosystems and their influence on the role of patients,” Business Research, vol. 13, no. 3, Sep. 2020, doi: https://doi.org/10.1007/s40685-020-00125-x.
- Massaro, “Digital transformation in the healthcare sector through blockchain technology. Insights from academic research and business developments,” Technovation, p. 102386, Sep. 2021, doi: https://doi.org/10.1016/j.technovation.2021.102386.
- Meske, I. Amojo, A.-S. Poncette, and F. Balzer, “The Potential Role of Digital Nudging in the Digital Transformation of the Healthcare Industry,” Lecture Notes in Computer Science, pp. 323–336, 2019, doi: https://doi.org/10.1007/978-3-030-23538-3_25.
- Haggerty, “Healthcare and digital transformation,” Network Security, vol. 2017, no. 8, pp. 7–11, Aug. 2017, doi: https://doi.org/10.1016/s1353-4858(17)30081-8.
- Iyanna, P. Kaur, P. Ractham, S. Talwar, and A. K. M. Najmul Islam, “Digital transformation of healthcare sector. What is impeding adoption and continued usage of technology-driven innovations by end-users?,” Journal of Business Research, vol. 153, pp. 150–161, Dec. 2022, doi: https://doi.org/10.1016/j.jbusres.2022.08.007.
- Thakur and S. Lahiry, “Digital clinical trial: A new norm in clinical research,” Perspectives in Clinical Research, vol. 12, no. 4, p. 184, 2021, doi: https://doi.org/10.4103/picr.picr_278_20.
- A. of S. Medicine Engineering, and, H. and M. Division, B. on H. S. Policy, and F. on D. D. Translation Development, and, Virtual Clinical Trials: Challenges and Opportunities: Proceedings of a Workshop. National Academies Press, 2019. Accessed: Jul. 17, 2023. [Online]. Available: https://books.google.com.pk/books?hl=en&lr=&id=7T-3DwAAQBAJ&oi=fnd&pg=PR1&dq=The+digital+shift+in+the+healthcare+industry+Clinical+Trials+in+a+virtual+setting&ots=5WjWDYFLdE&sig=MK3-pwNCDLkijj6h68dzQMXiosQ#v=onepage&q&f=false
- Shae and J. J. P. Tsai, “On the Design of a Blockchain Platform for Clinical Trial and Precision Medicine,” IEEE Xplore, Jun. 01, 2017. https://ieeexplore.ieee.org/document/7980138 (accessed Sep. 26, 2020).
- Haleem, M. Javaid, R. Pratap Singh, and R. Suman, “Exploring the revolution in healthcare systems through the applications of digital twin technology,” Biomedical Technology, vol. 4, pp. 28–38, Dec. 2023, doi: https://doi.org/10.1016/j.bmt.2023.02.001.
- T. Inan et al., “Digitizing clinical trials,” npj Digital Medicine, vol. 3, no. 1, Jul. 2020, doi: https://doi.org/10.1038/s41746-020-0302-y.
- Coran et al., “Advancing the Use of Mobile Technologies in Clinical Trials: Recommendations from the Clinical Trials Transformation Initiative,” Digital Biomarkers, vol. 3, no. 3, pp. 145–154, Nov. 2019, doi: https://doi.org/10.1159/000503957.
- L. Ventola, “Virtual Reality in Pharmacy: Opportunities for Clinical, Research, and Educational Applications,” Pharmacy and Therapeutics, vol. 44, no. 5, pp. 267–276, May 2019, Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6487969/
- H. Vlake et al., “Reporting the early stage clinical evaluation of virtual-reality-based intervention trials: RATE-VR,” Nature Medicine, pp. 1–2, Dec. 2022, doi: https://doi.org/10.1038/s41591-022-02085-7.
- I. Rhon et al., “TIDieR-telehealth: precision in reporting of telehealth interventions used in clinical trials – unique considerations for the Template for the Intervention Description and Replication (TIDieR) checklist,” BMC Medical Research Methodology, vol. 22, no. 1, Jun. 2022, doi: https://doi.org/10.1186/s12874-022-01640-7.
- Vagg, S. Shanthikumar, D. Morrissy, W. W. Chapman, B. J. Plant, and S. Ranganathan, “Telehealth and virtual health monitoring in cystic fibrosis,” Current Opinion in Pulmonary Medicine, vol. 27, no. 6, pp. 544–553, Nov. 2021, doi: https://doi.org/10.1097/MCP.0000000000000821.
- A. Greenwood, S. A. Blozis, H. M. Young, T. S. Nesbitt, and C. C. Quinn, “Overcoming Clinical Inertia: A Randomized Clinical Trial of a Telehealth Remote Monitoring Intervention Using Paired Glucose Testing in Adults With Type 2 Diabetes,” Journal of Medical Internet Research, vol. 17, no. 7, p. e178, 2015, doi: https://doi.org/10.2196/jmir.4112.
- P. Benziger, M. D. Huffman, R. N. Sweis, and N. J. Stone, “The Telehealth Ten: A Guide for a Patient-Assisted Virtual Physical Examination,” The American Journal of Medicine, Jul. 2020, doi: https://doi.org/10.1016/j.amjmed.2020.06.015.
- W. Fisher, K. C. Luczynski, S. A. Hood, A. D. Lesser, M. A. Machado, and C. C. Piazza, “Preliminary findings of a randomized clinical trial of a virtual training program for applied behavior analysis technicians,” Research in Autism Spectrum Disorders, vol. 8, no. 9, pp. 1044–1054, Sep. 2014, doi: https://doi.org/10.1016/j.rasd.2014.05.002.
- W. Fisher et al., “A randomized clinical trial of a virtual‐training program for teaching applied‐behavior‐analysis skills to parents of children with autism spectrum disorder,” Journal of Applied Behavior Analysis, vol. 53, no. 4, pp. 1856–1875, Sep. 2020, doi: https://doi.org/10.1002/jaba.778.
- Pekmezaris et al., “The Impact of Remote Patient Monitoring (Telehealth) upon Medicare Beneficiaries with Heart Failure,” Telemedicine and e-Health, vol. 18, no. 2, pp. 101–108, Mar. 2012, doi: https://doi.org/10.1089/tmj.2011.0095.
- M. Zork, “Telehealth for the Management of Diabetes in Pregnancy,” Current Diabetes Reports, vol. 22, no. 8, pp. 365–369, Jul. 2022, doi: https://doi.org/10.1007/s11892-022-01476-x.
- Leung, “Using AI–ML to Augment the Capabilities of Social Media for Telehealth and Remote Patient Monitoring,” Healthcare, vol. 11, no. 12, p. 1704, Jan. 2023, doi: https://doi.org/10.3390/healthcare11121704.
- Singh, A. Naik., “Redefining Boundaries in Healthcare: An In-Depth Exploration of Telemedicine’s and Wireless Technology’s Role in Emergency Care and Disaster Management,” IJCTT Journal Volume-71 Issue-6, 2023, doi: 10.14445/22312803/IJCTT-V71I6P102
- OpenNeuro – Registry of Open Data on AWS
- C. Fortney et al., “Telemedicine-Based Collaborative Care for Posttraumatic Stress Disorder,” JAMA Psychiatry, vol. 72, no. 1, p. 58, Jan. 2015, doi: https://doi.org/10.1001/jamapsychiatry.2014.1575.
© Copyright 2023, All Rights Reserved. Use of this content signifies your agreement to the T&Cs of Unified Citation Journals
This abstract of Manuscript/Paper/Article is an open access Manuscript/Paper/Article distributed under the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/) which allows and permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited and accepted.
This communication and any documents, or files, attached to it, constitute an electronic communication within the scope of the Electronic Communication Privacy Act (https://it.ojp.gov/PrivacyLiberty/authorities/statutes/1285)
To citation of this article: Deepak Singh, Sreeram M, Sanju Mannumadam Venugopal, Suyash Bhogawar, Telehealth and Virtual Clinical Trials: The Interplay of Data Engineering and Cloud Technologies for a Digital-First Healthcare Approach Use, Unified Nursing Research, Midwifery & Women’s Health Journal