Dr. George Jacob
BDS (Rajiv Gandhi University of Heath Sciences)
MDS (Conservative Dentistry and Endodontics, Kerala University)
Managing Director, ALL FOR ROOTS ACADEMY, Kochi
Chief Faculty, GAEA CYNOSURE, Dubai
Co Faculty, Pro Dental Academy, London, UK
Co Faculty, Martignoni Academy, Rome, Italy
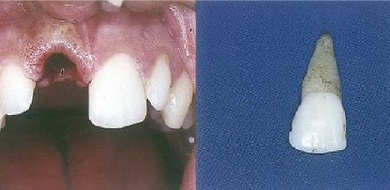
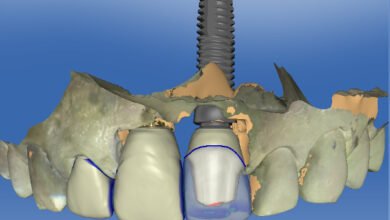
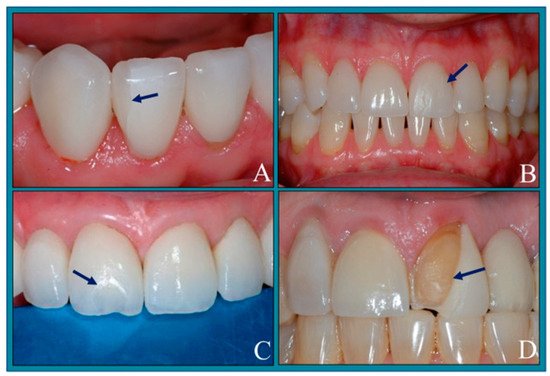
When we talk about Esthetic Dentistry round the world, the treatment modality what comes to our mind as a clinician is undoubtedly Porcelain Laminate Veneers. The latest advancements in digital dentistry and planning brings us to an accurate treatment with high patient satisfaction.
Porcelain Laminate Veneers for that matter is a highly technique sensitive therapy. In this lecture let’s delve into the key factors of success while executing this therapy on a day-to-day basis. Some basic clinical tips regarding smile designing will help the participants to take their practice to the next level. The success of these restorations greatly depends on the bonding protocol which will be emphasized in the lecture.
#PorcelainVeneers #LaminateVeneers #VeneerDentistry #CosmeticDentistry #EstheticDentistry #SmileDesign #DigitalSmileDesign #DSD #SmileMakeover #DentalAesthetics #DentalEsthetics #VeneerTreatment #SmileTransformation #SmileRehabilitation #DentalPractice #ModernDentistry #RestorativeDentistry #MinimallyInvasiveDentistry #AdhesiveDentistry #DentalCeramics #Prosthodontics #ConservativeDentistry #OralRehabilitation #SmileCare #AdvancedDentistry #SmileEnhancement #AestheticSmile #EstheticRevolution #PorcelainRestorations #DentalVeneers #SmileEsthetics #CeramicVeneers #CosmeticSmileDesign #DentalHealthAndAesthetics #SmileCrafting #DentalInnovation #SmileHarmony #SmileWithConfidence #VeneerSpecialist #SmileTreatment #DentalLaminate #OralEsthetics #ModernSmileSolutions #DentalTreatmentPlanning #BeautifulSmiles #SmileCareExperts #VeneerDentist #EstheticSolutions #ClinicalDentistry #AdvancedSmileDesign #ProfessionalDentistry