Metabolic Markers of Drug Naive Type 2 Diabetes Patients of Bangladesh: Study in Tertiary Care Hospitals

Global Journal of Diabetes, Endocrinology & Metabolic Disorders
Volume 1, Issue 1, September 2022, Pages: 22-33
Received: June 30, 2022, Reviewed: July 27, 2022; Accepted: August 8th, 2022; Published: September 08, 2022
Unified Citation Journals, Diabetes 20222, 1(2) 23-33; https://doi.org/10.52402/Diabetes703
ISSN: 2752-6283
Authors: Dr. Shahjada Selim*1, Dr. Mohammad Saifuddin2, Dr. Md. Abdul Hannan3, Dr. Muhammed Abu Bakar4, Dr. Samir Kumar Talukder5, Dr. A B M Kamrul Hasan6, Dr. Mohammad Nurul Amin7, Dr. Md. Azizul Hoque8, Md. Shahinur Rahman9, Dr. Ahmed Salam Mir10. Dr. Masud-Un Nabi11, Dr. Faria Afsana12
1Associate Professor, Department of Endocrinology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh
2Associate Professor, Diabetes & Endocrinology, Dhaka Medical College, Dhaka, Bangladesh
3Associate Professor, Department of Endocrinology, North East Medical College & Hospital, Sylhet, Bangladesh
4Assistant Professor Department of Endocrinology, Chattogram Maa O Shishu Hospital, Chattogram, Bangladesh
5Associate Professor, Department of Endocrinology, Rangpur Medical College, Rangpur, Bangladesh
6Assistant Professor & Head, Department of Endocrinology, Mymensingh Medical College, Mymensingh, Bangladesh.
7Assistant Professor, Department of Endocrinology, Mugda Medical College, Dhaka, Bangladesh
8Associate Professor, Endocrinology, Shaheed Ziaur Rahman Medical College, Bogura, Bangladesh
9Assistant Registrar, Department Of Diabetes and Endocrinology, Pabna Diabetic Association Hospital, Pabna, Bangladesh
10Associate Professor, Department of Endocrinology, Bangladesh Institute of Health Sciences
11Assistant Professor, Department of Endocrinology, Rajshahi Medical College, Rajshahi, Bangladesh
12Assistant Professor, Department of Endocrinology, BIRDEM, Dhaka, Bangladesh
Corresponding author: Dr. Shahjada Selim, Associate Professor, Department of Endocrinology, Bangabandhu Sheikh Mujib Medical University, Dhaka-1000, Bangladesh.
Abstract
Background: Almost all with type 2 diabetes are on one or the other drug along with therapeutic lifestyle interventions. However, it is very important to ascertain the clinical and biochemical findings of them to assess the treatment outcome and to determine and/or predict chronic complications. But there is a scarcity of data regarding drug naïve type diabetes patients evaluating biochemical profiles in Bangladesh.
Aim of the study: The aim of this study was to evaluate the metabolic marker’s assessment for drug-naive type 2 diabetes patients.
Methods: This was an open-label comparative observational real-life research that took place in the investigators’ chambers at numerous locations across Bangladesh in an outdoor environment from August to December 2020. A total of 250 individuals with drug-naive type 2 diabetes mellitus were recruited in the case group study. For the control group, another group of 30 healthy persons was chosen. Before beginning data collection, all participants signed a written permission form. Patent data was collected using a pre-designed questionnaire. MS Office and SPSS versions were used to handle, analyze, and disseminate all data as needed.
Result: In the case group, the mean (±SD) values of fasting glucose, mg/dL, 30-Minute glucose, mg/dL, fasting insulin, μIU/mL, 30-Min. insulin, μIU/mL, fasting C-peptide, ng/mL, and 30-Min. C-peptide, ng/mL were found 141.6±25.6, 233.6±21.3, 10.2±2.6, 17.4±3.2, 2.5±0.3 and 3.4±0.6 respectively. Besides these in the same group, the mean (±SD) values of the insulinogenic index, disposition index, HOMA-IR, and HbA1c, % were found 4.6±2.7, 0.16±0.01, 3.5±0.06, and 7.5±1.4 respectively. In this study in lipid profile analysis of the case group, we observed the mean values of total cholesterol, mg/dL, HDL-C, mg/dL, LDL-C, mg/dL, and triglycerides, mg/dL were 199.6±50.3, 47.1±12.8, 120.7±51.2 and 154.5±10.7 respectively. All the findings of metabolic markers of control group participants have been described in the table also. In that table, the P values regarding metabolic marker readings between both the case and control group patients have also been mentioned. In about all the metabolic marker’s comparisons, we found significant correlations between the groups (P<0.0001).
Conclusion: In about all the metabolic marker’s comparisons we found significant correlations between the groups. In treating drug-naive type 2 diabetes patients, analysis of metabolic marker’s findings may play an important role.
Keywords: Biochemical characteristics, Drug naive type 2 diabetes, Bangladesh.
INTRODUCTION: Type 2 diabetes mellitus (T2DM) is caused by a progressive decline of insulin secretion on the background of insulin resistance. T2DM encompasses around 90% of the patients with diabetes in the world. It is a purely multifactorial disorder. There is a role of genetic predisposition, but in many instances, T2DM is caused by environmental factors alone or in a combination of genetic factors. A sedentary lifestyle, consuming excess carbohydrates, weight gaining or obesity, smoking tobacco, excess salt intake, and environmental hazards are all implicated. Diabetes is becoming more common worldwide, but in South Asian nations, a sharp increase in diabetes incidence, particularly T2DM, has been reported.1 Unplanned urbanization, social insecurity, and rapid financial upgradation might be the important cause of that. The World Health Organization (WHO) predicts the number of people with diabetes to go up from 171 million (2000) to 366 million (2030) worldwide2. As per the International Diabetes Federation Atlas (2015), in the Southeast Asian region, 90 million people live with diabetes (prevalence: 8.7%). Increased life expectancy, increasing population growth, high rate of urbanization, low literacy, and inadequate national health expenditure are some reasons behind the higher prevalence of diabetes in this region3. Bangladesh is one of the six nations that make up the IDF Southeast Asia area. Diabetes affects 537 million adults worldwide, including 90 million in the Southeast Asian region alone, with a projected increase to 152 million by 2045.
In Bangladesh, diabetes affected about 11.6 million people in 2020 alone, with half of them undiagnosed.4 The Sodium-Glucose Co-Transporter-2 inhibitor (SGLT2i), which decreases renal glucose reabsorption, belongs to a new class of medications that target metabolic pathways unrelated to β cell function or insulin resistance. The European Medicines Agency (EMA) and Food and Drug Administration (FDA) have approved SGLT2i (canagliflozin, empagliflozin, and empagliflozin) as monotherapy and as add on to other antihyperglycemic agents. SGLT2i can benefit the diabetic population in many ways. SGLT2 not only controls Type 2 DM but also has other beneficial effects. Metformin is the recommended first-line pharmacological treatment for type 2 diabetes5. Metformin reduces hepatic glucose synthesis by inhibiting gluconeogenesis, and it also enhances glucose absorption in peripheral tissue.6 Metformin usually does not cause hypoglycemia per se and is a weight-neutral oral antidiabetic drug (OAD). In T2DM in cases of lower HbA1C, metformin monotherapy along with lifestyle management is usually initiated, but it generally failed to achieve optimal glycemic control. And if can, the glycemic status can not be maintained for a longer period.7 In such cases, there is often a failure to intensify treatment as appropriate8. In a retrospective cohort analysis of more than 81,000 patients with type 2 diabetes, the median time to add another oral glucose-lowering medication following a patient’s initial treatment was 1.6 to 2.9 years9. The reasons for this clinical inertia are unclear but likely include a reluctance to initiate more complex drug regimens10. Initial combination therapy with oral anti-diabetes drugs with complementary modes of action may provide more robust and durable glucose-lowering efficacy compared with the traditional stepwise approach11.
The American Diabetes Association/European Association for the Study of Diabetes and the Canadian Diabetes Association both propose a combined approach to first-line therapy for individuals with HbA1c levels of $9 percent ($75 mmol/mol) or $8.5 percent ($69 mmol/mol), respectively.5,12. Most of the glucose filtered by the kidney is reabsorbed by the sodium-glucose cotransporter 2 (SGLT2)13. SGLT2 inhibitors act by reducing renal glucose reabsorption, thereby increasing urinary glucose excretion and reducing hyperglycemia10. Empagliflozin is a potent and selective SGLT2 inhibitor11. When used as an add-on to metformin in patients with type 2 diabetes, empagliflozin (10 mg and 25 mg q.d.) significantly reduced HbA1c, fasting plasma glucose (FPG), and weight and was well tolerated, with a low risk of hypoglycemia14. This study was undertaken to determine the biochemical parameters of drug-naive type 2 diabetes patients in Bangladesh.
METHODOLOGY & MATERIALS: This was an open-label comparative observational real-life study that was conducted in the chambers of the investigators in several places of Bangladesh in an outdoor setting during the period from August 2020 to December 2020. In total, 250 patients with drug-naive type 2 diabetes mellitus were enrolled as the study population for the case group. Other groups of 30 healthy people were selected for the control group. Properly written consent was taken from each of the participants before starting data collection. A pre-designed questionnaire was used for patient data collection. Patients with type 1 diabetes, a history of urinary tract infection more than three times in the preceding 12 months, patients with a recent (in the last three months) genital mycotic infection, and those who were pregnant or expecting to become pregnant were all excluded from this study. The investigator has examined the patients to ensure that they meet the eligibility requirements. A preset questionnaire format was used to capture a detailed medical history, including baseline laboratory values, clinical assessments, and medical information (Patient file). Standard prescriptions with drug names and dose instructions were also kept track of. Patients came in for a follow-up visit after one week. The investigator gave suggestions to patients regarding disease and medication (If any). Two follow-up visits had been done at 3 months intervals.
The patients had been asked to perform some predefined laboratory tests and bring those results in subsequent follow-up visits. Subjects would have been given a diary. Patients noted any Adverse Events (AE) that occurred over the three-month period. They would request that any Adverse Events be reported to the appropriate Investigator immediately or over the phone. The investigator also gave patients advice over the phone or, if required, requested a chamber visit. The health professionals had provided them a reminder one day before their planned visit. Patients returned to the chamber for a follow-up appointment (three months later) with the results of the recommended laboratory investigations. In the patient-specific questionnaire, the reports were collected alongside clinical assessments and diary card evaluations. The 3rd visit was conducted after 3 months after the 2nd visit (6 months after the enrolment). The same process had been applied to all patients by respective investigators. Each investigator handled 8 (8 for each arm, 8×3=24 in total) patients and there were 14 investigators so a total of 50 patients’ data had been generated. Clinical laboratory data and adverse events (AEs) were used as safety endpoints, with preferred words recorded using the Medical Dictionary for Drug Regulatory Activities (MedDRA) version 23.0.
AE’s included all events with an onset after the first dose and up to 7 days after the last dose of trial medication. Confirmed hypoglycemic AEs, defined as AEs with plasma glucose, 70 mg/dL (3.9 mmol/L) and/or requiring assistance, were identified by direct plasma glucose measurements and by home blood glucose monitoring performed by the patient. Other predefined AEs of special interest included events consistent with urinary tract infection (UTI), genital infection, and volume depletion, identified using prospectively defined search categories based on 67, 87, and 8 MedDRA preferred terms, respectively. A post hoc search for the MedDRA recommended keywords pollakiuria, polyuria, and nocturia was used to examine AEs linked to increased urination. SPSS was used to examine the data on the computer (Statistical Package for Social Science). Statistical analyses had been done by using appropriate statistical tools like the ‘chi-square’ test, and the student’s ‘T-test, where applicable. Statistical significance was set at 0.05 level and confidence interval at 95% level. Data had been presented in the form of tables and graphs.
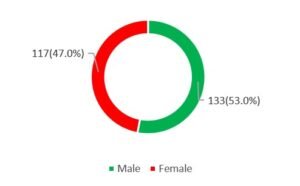
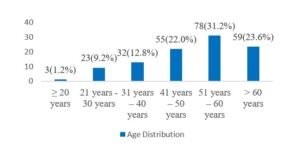
RESULT: In this study 53% (n=133) of the respondents were male whereas 47% (n=117) were female. So, male dominance was observed in number, and the male-female ratio was 1.4:1. In this study in analyzing the ages of the participants we observed, the highest number of participants were from the 51-60 years age group which was 31.20%. Besides this 1.20%, 9.20%, 12.80%, 22.00% and 23.60% were from ≥20, 21-30, 31-40, 41-50 and >60 years’ age groups respectively. In analyzing the other demographic status of the participants, we observed, the mean (±SD) Age (Year), body mass index (kg/m2), waist circumference (cma), systolic blood pressure (mm Hg), and diastolic blood pressure, (mm Hg) were 51.7±10.5, 25.6±3.3, 87.9±9.1, 130.4±14.1 and 82.6±9.8 respectively. In this study, the use of antihypertensive drugs, use of lipid-lowering drugs, metabolic syndrome, dietary therapy, physical activity, current smoker, and current drinker were found positive among 37.20%, 24.00%, 69.20%, 14.00%, 26.80%, 42.80% and 10.80% participants respectively.
In this study, in the case group the mean (±SD) values of fasting glucose, mg/dL, 30-Minute glucose, mg/dL, fasting insulin, μIU/mL, 30-Min. insulin, μIU/mL, fasting C-peptide, ng/mL, and 30-Min. C-peptide, ng/mL were found 141.6±25.6, 233.6±21.3, 10.2±2.6, 17.4±3.2, 2.5±0.3 and 3.4±0.6 respectively. Besides these in the same group, the mean (±SD) values of the insulinogenic index, disposition index, HOMA-IR, and HbA1c, % were found to be 4.6±2.7, 0.16±0.01, 3.5±0.06, and 7.5±1.4 respectively. In this study in lipid profile analysis of the case group, we observed the mean values of total cholesterol, mg/dL, HDL-C, mg/dL, LDL-C, mg/dL, and triglycerides, mg/dL were 199.6±50.3, 47.1±12.8, 120.7±51.2 and 154.5±10.7 respectively. All the findings of metabolic markers of control group participants have been described in the table also. In that table, the P values regarding metabolic marker readings between both the case and control group patients have also been mentioned. In about all the metabolic marker’s comparisons we found significant correlations between the groups (P<0.0001).
Figure I: Sex distribution of the participants (n=250)
Figure II: Age distribution of case group participants (n=250)
Table I: Demographic status of case group in mean ± SD (n=250)
| Characteristic | Mean ± SD |
| Age (Year) | 51.7±10.5 |
| Body mass index, kg/m2 | 25.6±3.3 |
| Waist circumference, cma | 87.9±9.1 |
| Systolic blood pressure, mm Hg | 130.4±14.1 |
| Diastolic blood pressure, mm Hg | 82.6±9.8 |
Table II: Medication, DM of case group participants (n=250)
| Characteristics | n (%) |
| Use of antihypertensive drugs | 93 (37.20) |
| Use of lipid-lowering drugs | 60 (24.00) |
| Metabolic syndrome | 173 (69.20) |
| Dietary therapy | 35 (14.00) |
| Physical activity | 67 (26.80) |
| Current smoker | 107 (42.80) |
| Current drinker | 27 (10.80) |
III: Mean and SD between the Case and Control groups (n=280)
| Characteristics | Case (n=250) | Control (n=30) | P value |
| Fasting glucose, mg/dL | 141.6±25.6 | 96.5±17.2 | <0.0001 |
| 30-Minute glucose, mg/dL | 233.6±21.3 | 132.1±19.7 | <0.0001 |
| Fasting insulin, μIU/mL | 10.2±2.6 | 6.7±3.1 | <0.0001 |
| 30-Min. insulin, μIU/mL | 17.4±3.2 | 24.2±7.4 | <0.0001 |
| Fasting C-peptide, ng/mL | 2.5±0.3 | 5.6±2.1 | <0.0001 |
| 30-Min. C-peptide, ng/mL | 3.4±0.6 | 0.6±0.1 | <0.0001 |
| Measures of β-cell function of patients: mean (Range) | |||
| Insulinogenic index | 4.6±2.7 | 2.1±0.7 | <0.0001 |
| Disposition index | 0.16±0.01 | 1.3±0.3 | <0.0001 |
| HOMA-IR | 3.5±0.06 | 1.1±0.2 | <0.0001 |
| HbA1c, % | 7.5±1.4 | 4.9±1.4 | <0.0001 |
Table IV: Mean and SD readings between the Case and Control groups of Lipid Profile (n=280)
| Characteristics | Case (n=250) | Control (n=30) | P value |
| Total cholesterol, mg/dL | 199.6±50.3 | 147.1±38.4 | <0.0001 |
| HDL-C, mg/dL | 47.1±12.8 | 49.7±13.4 | 0.296 |
| LDL-C, mg/dL | 120.7±51.2 | 91.7±15.8 | 0.002 |
| Triglycerides, mg/dL | 154.5±10.7 | 138.4±21.7 | <0.0001 |
DISCUSSION: The aim of this study was to metabolic marker assessment for drug-naive type 2 diabetes patients. In this study in the case group the mean (±SD) values of fasting glucose, mg/dL, 30-Minute glucose, mg/dL, fasting insulin, μIU/mL, 30-Min. insulin, μIU/mL, fasting C-peptide, ng/mL, and 30-Min. C-peptide, ng/mL were found 141.6±25.6, 233.6±21.3, 10.2±2.6, 17.4±3.2, 2.5±0.3 and 3.4±0.6 respectively. Besides these in the same group, the mean (±SD) values of the insulinogenic index, disposition index, HOMA-IR, and HbA1c, % were found 4.6±2.7, 0.16±0.01, 3.5±0.06, and 7.5±1.4 respectively. In this study in lipid profile analysis of the case group, we observed the mean values of total cholesterol, mg/dL, HDL-C, mg/dL, LDL-C, mg/dL, and triglycerides, mg/dL were 199.6±50.3, 47.1±12.8, 120.7±51.2 and 154.5±10.7 respectively. A study conducted at BIRDEM hospital in Dhaka, Bangladesh found 60.6% uncontrolled diabetes which was lower than our findings15. A rural hospital-based study in Gujarat, India showed that 80.7% of participants had uncontrolled diabetes16. One Pakistani study found that 46.2% of the patients with diabetes fail to achieve good glycemic control (HbA1C<7%).17 Among the factors of failure in diabetes control inadequate diabetes education, poor dietary management & physical activity, use of suboptimal drugs and therapeutic inertia have been identified.17
The findings suggest poor glycemic control and suboptimal diabetes management, which does not sufficiently reach the patients. Measures to improve clinic attendance, medication adherence, awareness, and ability to manage diabetes are needed. Insulin initiation is usually delayed by several years and whenever initiated, not with the modern one. Here patient’s education, financial, logistic barriers, and lack of government support might have contributed. Diabetes is responsible for an estimated 6.7 million extra adult deaths worldwide, with CVD accounting for over half of these fatalities and renal failure accounting for around 10%18. In a 2003 research at a big tertiary diabetes hospital in Dhaka, 72 percent of diabetic patients presented with complications such as cardiovascular disease (31.8 %), retinopathy (13.8 %), nephropathy (5.84 %), and neuropathy (4.55 %)19. Retinopathy, nephropathy, and neuropathy were observed in 12.9 percent, 38.8%, and 2.4 percent of diabetic individuals in a Chinese investigation19. According to research in Rajshahi, Bangladesh, 21% of people have cataracts, 14% have diabetic nephropathy, 35% have neurological disorders, and 6% have renal problems20. Eye issues were the most common complication in this research, followed by CVD, CKD, neurological difficulties, and others. Another study in Bangladesh found that 49.7% of patients with diabetes had complications which were lower than our study15. Because this was a cross-sectional study, current baseline HbA1c, FPG, and 2hAFB values were utilized to forecast the onset of diabetic problems that had occurred during the illness and might differ from our present results. Furthermore, no precise or defined measures of macrovascular and microvascular problems were reported in this investigation, and data was gathered via records review. Furthermore, the participants in this study were T2D patients on oral drug therapy who were relatively new cases, therefore they may not represent all diabetics. All the findings of metabolic markers of control group participants have been described in the table also. In that table, the P values regarding metabolic marker’s readings between both the case and control group patients have also been mentioned. In about all the metabolic marker’s comparisons we found significant correlations between the groups (P<0.0001).
Limitations of the study
This was an observational study with a small-sized sample. So, the findings of this study may not reflect the exact scenario of the whole country.
CONCLUSION AND RECOMMENDATIONS: In about all the metabolic marker’s comparisons, we found significant correlations between the groups. In treating drug-naive type 2 diabetes patients, analysis of metabolic marker’s findings may play an important role. For getting more reliable information we would like to recommend conducting more studies in several places with larger sized samples.
Conflict of Interest: None declared
REFERENCES:
- Walaa Mohammedsaeed, Saiema Ahmedi, Nikhat Manzoor; Understanding the Interplay between COVID-19 and Diabetes Mellitus,Journal of Biosciences and Medicines 2020,8:1-12
- Sanjay Kalra, Sujoy Ghosh, A. H. Aamir, Md. Tofail Ahmed, Mohammod Feroz Amin, Sarita Bajaj, Manash P. Baruah, Uditha Bulugahapitiya, A. K. Das, Mimi Giri, Sonali Gunatilake, Saeed A.Mahar, Md. Faruque Pathan, Nazmul Kabir Qureshi, S. Abbas Raza, Rakesh Sahay, Santosh Shakya, Dina Shreshta, Noel Somasundaram, Manilka Sumanatilleke, A. G.Unnikrishnan1,Achini Madushani Wijesinghe: Safe and pragmatic use of sodium–glucose co‑transporter 2 inhibitors in type 2 diabetes mellitus: South Asian Federation of Endocrine Societies consensus statement, 2017 Indian Journal of Endocrinology and Metabolism 2017 Jan-Feb; 21(1):210-230
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27:1047-53.
- Banik PC, Mondal R, Hussain A. Overweight and obesity among the urban and rural type 2 diabetic subjects in Bangladesh.
- Hundal RS, Krssak M, Dufour S, et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 2000; 49:2063–2069
- Basu R, Shah P, Basu A, et al. Comparison of the effects of pioglitazone and metformin on hepatic and extra-hepatic insulin action in people with type 2 diabetes. Diabetes 2008; 57:24–31
- Turner RC, Cull CA, Frighi V, Holman RR; UK Prospective Diabetes Study (UKPDS) Group. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA 1999; 281:2005–2012
- Triplitt C. Improving treatment success rates for type 2 diabetes: recommendations for a changing environment. Am J Manag Care 2010;16(Suppl.): S195–S200
- Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care 2013; 36:3411–3417
- Miccoli R, Penno G, Del Prato S. Multidrug treatment of type 2 diabetes: a challenge for compliance. Diabetes Care 2011;34(Suppl. 2): S231–S235
- DeFronzo RA, Eldor R, Abdul-Ghani M. Pathophysiologic approach to therapy in patients with newly diagnosed type 2 diabetes. Diabetes Care 2013;36(Suppl. 2): S127–S138
- Cheng AY; Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2013 clinical practice guidelines for the prevention and management of diabetes in Canada. Introduction. Can J Diabetes 2013;37(Suppl. 1): S1–S3
- Gallo LA, Wright EM, Vallon V. Probing SGLT2 as a therapeutic target for diabetes: basic physiology and consequences. Diab Vasc Dis Res 2015; 12:78–89
- Grempler R, Thomas L, Eckhardt M, et al. mpagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: characterization and comparison with other SGLT-2 inhibitors. Diabetes Obes Metab 2012; 14:83–90
- Islam MR, Islam MS, Karim MR, Alam UK, Yesmin KIslam MR, Islam MS, et al. Predictors of diabetes distress in patients with type 2 diabetes mellitus. Int J Res Med Sci 2 2014;631–8.
- Pandya H, Lakhani JD, Dadhania J, Trivedi A. The prevalence and pattern of dyslipidemia among type 2 diabetic patients at rural based hospital in Gujarat, India. Indian J Clin Pract 2012;22(12):36–44.
- Akbar DH, Al-Gamdi AA, Hejazi NA. Poor lipid control in type-2 diabetics with and without ischemic heart disease. Endocrine 2003;21(3):217–20.
- Beulens JW, Grobbee DE, Nealb B. The global burden of diabetes and its complications: an emerging pandemic. Eur J Cardiovasc Prev Rehabil 2010;17(1 suppl): s3–8.
- Islam MZ. Disability burden of diabetes mellitus and its complications. J Med 2009;10(3):22–6.
- Sultana Z, Ali M, Uddin M, Haque M. A study of evaluation for the management of diabetes in Bangladesh. Pharmacol Pharm 2013;4(3).
Copyright
© Copyright 2022, All Rights Reserved. Use of this content signifies your agreement to the T&Cs of Unified Citation Journals
This abstract of Manuscript/Paper/Article is an open access Manuscript/Paper/Article distributed under the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/) which allows and permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited and accepted.
This communication and any documents, or files, attached to it, constitute an electronic communication within the scope of the Electronic Communication Privacy Act (https://it.ojp.gov/PrivacyLiberty/authorities/statutes/1285)
To citation of this article: Dr. Shahjada Selim*1, Dr. Mohammad Saifuddin2, Dr. Md. Abdul Hannan3, Dr. Muhammed Abu Bakar4, Dr. Samir Kumar Talukder5, Dr. A B M Kamrul Hasan6, Dr. Mohammad Nurul Amin7, Dr. Md. Azizul Hoque8, Md. Shahinur Rahman9, Dr. Ahmed Salam Mir10. Dr. Masud-Un Nabi11, Dr. Faria Afsana12, Metabolic Markers of Drug Naive Type 2 Diabetes Patients of Bangladesh: Study in Tertiary Care Hospitals, Global Journal of Diabetes, Endocrinology & Metabolic Disorders