A case study of liraglutide in an obese diabetic patient in a District General Hospital
Global Journal of Diabetes, Endocrinology & Metabolic Disorders
Volume 1, Issue 1, June 2020, Pages: 12-15
Received: Aug 8, 2020, Reviewed: Aug 31, 2020; Accepted: Oct 1, 2020; Published: April 1, 2021
Unified Citation Journals, Diabetes 2021, 1(2) 12-15; https://doi.org/10.52402/Diabetes701
ISSN: 2752-6283
Authors: Dr. Mohammed Ashfaqul Ghani![]() , Dr. Christopher UY, Dr. Aye Thet Hlyar Oo
, Dr. Christopher UY, Dr. Aye Thet Hlyar Oo
Eastbourne District General Hospital
*Author to whom correspondence should be addressed.
Download PDFAbstract:
Introduction:
Diabetes mellitus is increasing rapidly worldwide, and treatment comes with many challenges. It is estimated that in 2030, approximately 500 million people will live with diabetes across the world—most of which will be type 2 diabetes mellitus (T2DM). Obesity often coincides with T2DM and has been linked to increased insulin resistance, high blood pressure, and high blood lipids. Thus, pharmacological management should be aimed at promoting weight loss or at very least be weight neutral. In many cases, the risks of hypoglycaemia and weight gain may delay titration of diabetic agents to HbA1C targets.
Both insulin and sulfonylureas are proven medications that are beneficial for tight glycemic control but with an increased risk of weight gain and hypoglycaemia. Newer agents under the drug class glucagon-like peptide receptor antagonists (GLP-1RA) are increasingly being used. Various randomized clinical trials support that obese T2DM patients treated with GLP-1RA lead to better glycaemic control, weight reduction, and reduced risk of hypoglycaemia.
Keywords: T2DM, Liraglutide, GLP-1 RA, Obesity
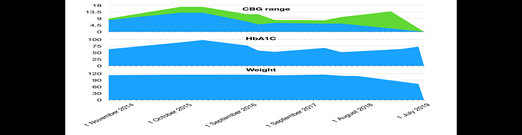
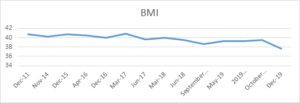
Case Summary: A 56-year-old female was diagnosed with T2DM in 2003 and had been regularly followed up in a diabetes clinic since 2014. During the initial clinic review, she had a bodyweight of 114.9 kg (BMI 40.7), fasting blood glucose was 8 – 9, 2-hour CBG 11-13, and HbA1c of 87. At that time, she was taking insulin glargine (Lantus) 36 units once daily, gliclazide 40 mg in the morning / 120 mg in the evening, metformin slow release 2 g in the evening, and simvastatin 20 mg at bedtime. After discussion with her, she was started on the GLP-1RA liraglutide subcutaneously. She had no diabetic-related complications. She continued liraglutide since then. Her HbA1c started to improve and also noticed a change in body weight which had gradually decreased from 114.9 to 109 kg. Her liraglutide was held at the latter end of 2018 as her glucose control and weight had been maintained. It was noticed that after stopping liraglutide her blood glucose and weight started to go up again even though her other medications remained the same. Her case was discussed at Diabetes MDT and liraglutide1.8mg restarted in September 2019 and since then she able to lose around 5% of her body weight and HBA1C also started to drop.
It is noticed that since the starting of her liraglutide her HBA1C level and weight fall by around 1% and 4% respectively. In late 2018 once she stopped liraglutide her HbA1C and weight started to rise again. In 2019 liraglutide was restarted and she managed to reduce body weight by 5kg and HbA1C by 2%. During the whole time period, she maintained lifestyle intervention.

Discussion:
Excessive fat accumulation with potential impairing effects on health know as overweight and obesity is a major risk factor for type 2 diabetes mellitus. Most T2DM people around 80-90% fall in mild to moderate obesity or overweight need either behavior or medication-based weight loss programme. In these patients losing as little as 5% of body weight positively affect their cardiovascular mortality and glycemic control. There is no need to mention that losing body weight and maintaining it is a challenge for the majority of diabetic patients and it is especially true for those who are on oral hypoglycaemic agents such as insulin, sulfonylurea, and thiazolidinediones, and insulin. In that case GLP-! Receptor agonists (GLP-1 Ras) is exceptional and it is proven benefit in reducing weight in T2DM obese patients.
Liraglutide is first approved in 2010 as an adjunct therapy to diet and exercise for the management of type 2 diabetes. Liraglutide is a derivative of GLP-1, a polypeptide incretin hormone secreted by the L-cell of the gastrointestinal tract. It stimulates glucose-dependent insulin secretion causing a decrease in plasma glucagon concentrations, delayed gastric emptying, suppress appetite, and increased heart rate. It is believed that the weight-lowering effect of GLP-1RA is due to appetite suppression and delayed gastric emptying.
After liraglutide administration peak absorption occurs at 11 hours and its absolute bioavailability is 55%. Its half-life in 13 hours, allowing it once-daily administration. It eliminates through the liver and kidneys and does not interfere with cytochrome p450 system. Most common side effects are nausea, hypoglycaemia, diarrhoea, constipation, abdominal pain and increased serum lipase. Gastrointestinal intolerance is the most common reason for drug discontinuation in patients. There is also an increased correlation with acute pancreatitis, serious hypoglycaemic episodes, tachycardia, and suicidal behaviour. Liraglutide is contraindicated in pregnancy and should be avoided in nursing mothers, children, and coincident use with other GLP-1 agonists.
Copyright
© Copyright 2021, All Rights Reserved. Use of this content signifies your agreement to the T&Cs of Unified Citation Journals
This abstract of Manuscript/Paper/Article is an open access Manuscript/Paper/Article distributed under the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/) which allows and permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited and accepted.
This communication and any documents, or files, attached to it, constitute an electronic communication within the scope of the Electronic Communication Privacy Act (https://it.ojp.gov/PrivacyLiberty/authorities/statutes/1285)
To citation of this article: Dr. Mohammed Ashfaqul Ghani, A case study of liraglutide in an obese diabetic patient in a District General Hospital, Global Journal of Diabetes, Endocrinology & Metabolic Disorders
References:
- Gan, S., Dawed, A., Donnelly, L., Nair, A., Palmer, C., Mohan, V. and Pearson, E., 2021. Efficacy of Modern Diabetes Treatments DPP-4i, SGLT-2i, and GLP-1RA in White and Asian Patients With Diabetes: A Systematic Review and Meta-analysis of Randomized Controlled Trials.
- Mehta, A., Marso, S. and Neeland, I., 2021. Liraglutide for weight management: a critical review of the evidence.
- Montanya, E. and Sesti, G., 2021. A review of efficacy and safety data regarding the use of liraglutide, a once-daily human glucagon-like peptide 1 analogue, in the treatment of type 2 diabetes mellitus.
- Mirabelli, M., Chiefari, E., Caroleo, P., Arcidiacono, B., Corigliano, D., Giuliano, S., Brunetti, F., Tanyolaç, S., Foti, D., Puccio, L. and Brunetti, A., 2021. Long-Term Effectiveness of Liraglutide for Weight Management and Glycemic Control in Type 2 Diabetes.